Benzene
Molecular Structure: Benzene is a six-membered carbon ring with alternating single and double bonds, represented as C6H6. The structure involves a resonance hybrid, with delocalized pi electrons over the entire ring.

Aromatic Properties: Benzene is an aromatic hydrocarbon, characterized by its high stability and resistance to typical alkene reactions. The stability arises from the delocalization of pi electrons.
Physical Properties: It is a colorless liquid with a characteristic sweet odor, and it has a boiling point of 80.1 degrees Celsius and a melting point of 5.5 degrees Celsius.
Common Benzene Derivatives
1. Toluene:
Structure: Toluene is a benzene derivative with a methyl group (-CH3) substituent. Its IUPAC name is methylbenzene.
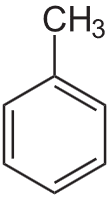
Use: It is used as a solvent in producing explosives and synthesizing various chemicals.
2. Phenol:
Structure: Phenol is benzene with an attached hydroxyl group (-OH). Its IUPAC name is hydroxybenzene.

Use: Phenol is used in producing plastics, resins, and pharmaceuticals. It also has antiseptic properties.
3. Aniline:
Structure: Aniline is benzene with an amino group (-NH2) attached. Its IUPAC name is aminobenzene.
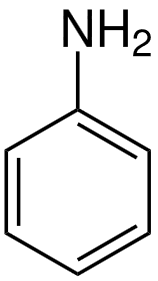
Use: Aniline synthesizes dyes, pharmaceuticals, and rubber chemicals.
4. Benzaldehyde:
Structure: Benzaldehyde is benzene with a formyl group (-CHO) attached. Its IUPAC name is benzenecarbaldehyde.

Use: It produces dyes, perfumes, and flavoring agents.
5. Benzene Sulfonic Acid:
Structure: Benzene sulfonic acid has a sulfonic acid group (-SO3H) attached to benzene. Its IUPAC name is benzenesulfonic acid.

Use: It is used to synthesize detergents, pharmaceuticals, and dyes.
Reactions of Benzene and Its Derivatives
1. Electrophilic Aromatic Substitution:
Benzene and its derivatives undergo electrophilic aromatic substitution reactions where an electrophile replaces a hydrogen atom on the benzene ring.
2. Friedel-Crafts Reactions:
Friedel-Crafts alkylation and acylation reactions involve introducing alkyl or acyl groups onto the benzene ring in the presence of a Lewis acid catalyst.
3. Nitration:
Nitration of benzene involves the substitution of a hydrogen atom with a nitro group (-NO2) in the presence of nitric acid and sulfuric acid.
4. Halogenation:
In the presence of a halogen carrier, benzene undergoes halogenation reactions, wherein a halogen (e.g., chlorine or bromine) replaces a hydrogen atom.
Environmental and Health Considerations
– Benzene is a known carcinogen, and prolonged exposure can lead to serious health issues. Therefore, there are strict regulations regarding its industrial use and environmental release.
– Benzene derivatives may exhibit varying toxicity and environmental impact, depending on the specific substituents attached to the benzene ring.
Understanding benzene’s properties, reactions, and applications and its derivatives is essential in organic chemistry, industry, and environmental science. While benzene is highly stable and less reactive, its derivatives play crucial roles in various industrial processes and the synthesis of important chemicals.




4 Replies to “Benzene and Its Derivatives”