Qualitative Tests for Phenols: Phenols are aromatic compounds in which a hydroxyl (-OH) group is directly attached to the benzene ring. Due to their acidic nature and unique chemical properties, phenols can be identified using various qualitative tests. These tests are based on the reaction of phenols with specific reagents, leading to color changes or the formation of precipitates. Below are some of the most common qualitative tests for phenols:
Qualitative Tests for Phenols
1. Ferric Chloride Test
Principle:
Phenols react with neutral ferric chloride (FeCl3) to form colored complexes due to the formation of iron-phenol chelates.
Procedure:
- Dissolve the phenol sample in water or alcohol.
- Add a few drops of freshly prepared neutral ferric chloride solution (FeCl3).
- Observe the color change.
Observation:
- A violet, blue, green, or red color indicates the presence of phenols.
- Different phenols may give different colors depending on their structure.
Reaction:
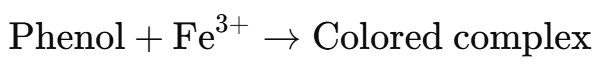
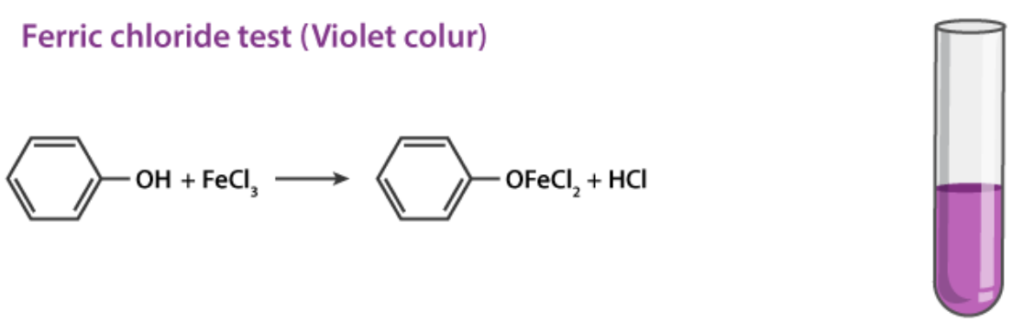
Example:
Phenol itself gives a violet color.
Catechol gives a green color.
Pyrogallol gives a brownish-red color.
2. Bromine Water Test
Principle: Phenols react with bromine water to form white precipitates of polybrominated phenols due to the high reactivity of the hydroxyl group in the benzene ring.
Procedure:
- Take a small amount of phenol in water.
- Add bromine water dropwise with continuous shaking.
- Observe the formation of a precipitate.
Observation:
A white precipitate of 2,4,6-tribromophenol indicates the presence of phenol.
Reaction:
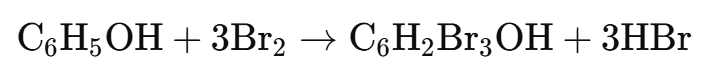
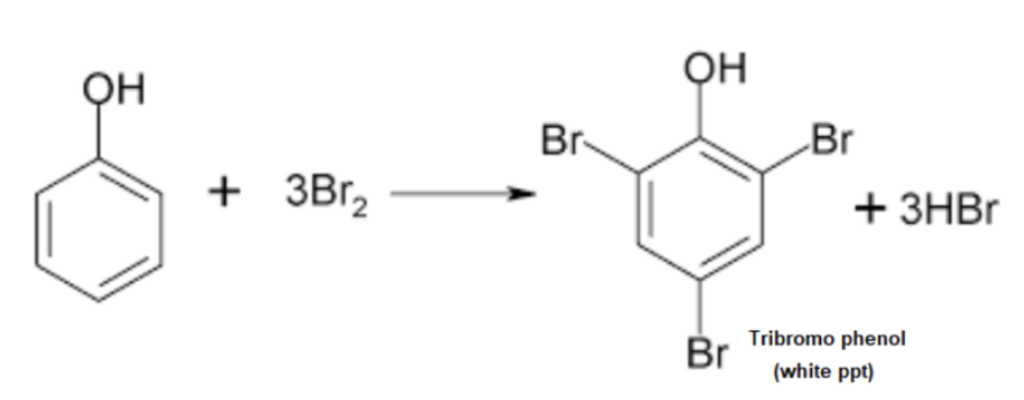
Example: Phenol gives a white precipitate of 2,4,6-tribromophenol.
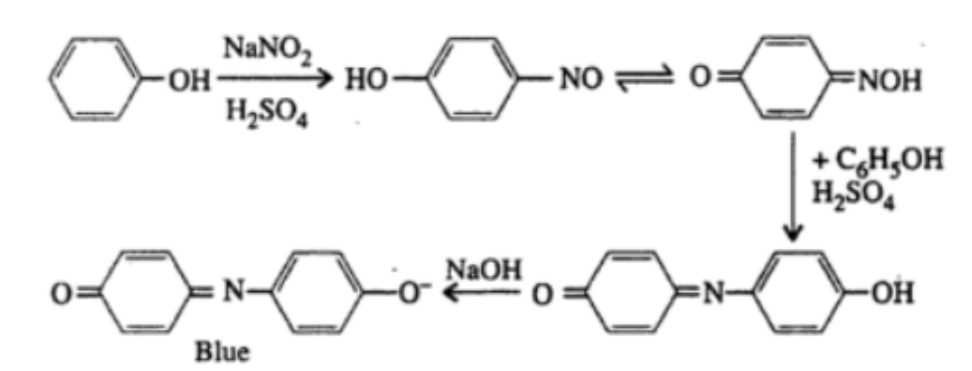
3. Liebermann’s Test
Principle: Phenols react with sodium nitrite (NaNo2) in the presence of concentrated sulfuric acid (H2So4) to give a deep green or blue color, which turns red upon dilution with water and then blue again upon making the solution alkaline.
Procedure:
- Take a small amount of phenol.
- Add concentrated sulfuric acid and a few crystals of sodium nitrite.
- Observe the color change.
- Add water and check if the color turns red.
- Add sodium hydroxide (NaOH) solution and check if the color turns blue again.
Observation: The appearance of a deep green or blue color that changes to red and back to blue confirms the presence of phenols.
4. Phthalein Dye Test
Principle: Phenols react with phthalic anhydride in the presence of concentrated sulfuric acid to form phthalein dyes, which show a pink color in alkaline conditions.
Procedure:
- Mix phenol with phthalic anhydride.
- Add concentrated sulfuric acid and heat the mixture.
- Cool and pour into an aqueous sodium hydroxide solution.
- Observe the color change.
Observation: The development of a pink or red color in an alkaline medium indicates the presence of phenol.
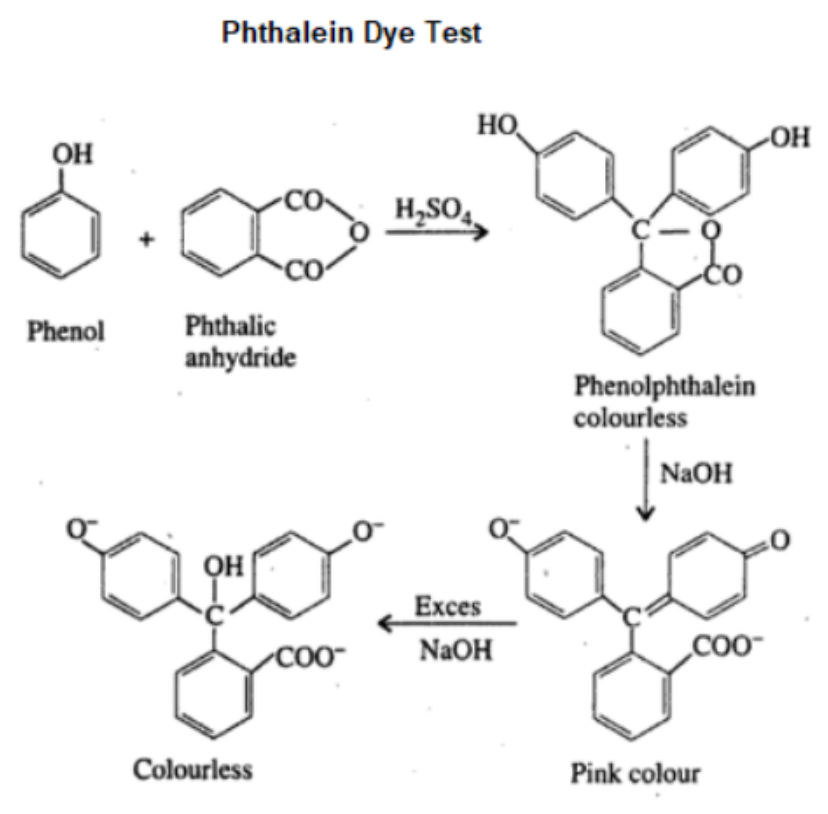
Example: Phenol forms phenolphthalein, which turns pink in alkaline conditions.
5. Reimer-Tiemann Reaction
Principle: Phenols react with chloroform (CHCl3) in the presence of aqueous sodium hydroxide (NaOH) to form ortho-hydroxy benzaldehyde (salicylaldehyde).
Procedure:
- Dissolve phenol in aqueous sodium hydroxide.
- Add chloroform dropwise and heat the mixture.
- Observe the formation of an aromatic aldehyde.
Observation: The presence of a characteristic odor of salicylaldehyde confirms the presence of phenol.
Reaction:
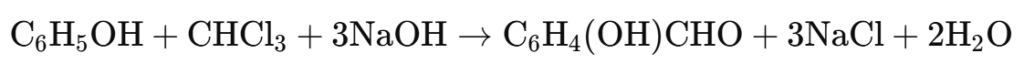
6. Azo Coupling Reaction
Principle: Phenols react with diazonium salts to form colored azo compounds due to electrophilic substitution at the para position of the benzene ring.
Procedure:
- Prepare a diazonium salt by reacting an aromatic amine (like aniline) with sodium nitrite (NaNO2) and hydrochloric acid (HCl).
- Add phenol to this solution.
- Observe the color change.
Observation: The formation of a yellow, orange, or red-colored azo compound indicates the presence of phenol.
7. Acylation Reaction (Esterification)
Principle: Phenols react with acetyl chloride (CH3COCl) or acetic anhydride ((CH3CO)2O) to form esters, which can be detected by their characteristic smell.
Procedure:
- Take phenol in a test tube.
- Add acetyl chloride or acetic anhydride.
- Observe the formation of an ester.
Observation: The presence of an ester with a characteristic fruity odor indicates the presence of phenol.
Conclusion
Phenols can be qualitatively detected using various chemical tests. Among these, the Ferric Chloride Test and the Bromine Water Test are the most commonly used due to their high specificity and ease of execution. Each test exploits a different chemical property of phenols, making them valuable tools in organic chemistry for the identification of phenolic compounds.
Visit to: Pharmacareerinsider.com

