axial hydrogen and equatorial hydrogen
axial hydrogen and equatorial hydrogen: In cyclohexane, a common cyclic hydrocarbon, two types of hydrogens are present due to the chair conformation of the molecule: axial hydrogens and equatorial hydrogens.
1. Axial Hydrogens: Equatorial hydrogens are oriented approximately along the plane of the cyclohexane ring, extending outward from the carbon atoms. These hydrogens are perpendicular to the imaginary axis passing through the center of the ring. Due to their outward orientation, equatorial hydrogens experience less steric hindrance and reduced repulsion from neighboring groups. As a result, they are generally more stable compared to axial hydrogens. In substituted cyclohexane derivatives, bulkier groups prefer the equatorial position to minimize steric strain and achieve a more stable conformation.
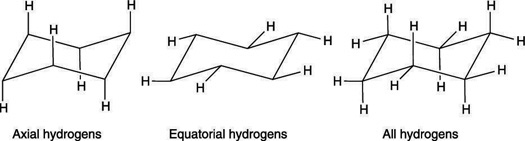
2. Equatorial Hydrogens: Equatorial hydrogens are those that are oriented approximately along the horizontal plane of the cyclohexane ring. They extend outward from the carbon atoms, projecting away from the ring structure in a direction roughly perpendicular to the imaginary axis passing through the center of the molecule. This positioning makes equatorial hydrogens more spatially extended compared to axial hydrogens, reducing their likelihood of steric interactions with other substituents on the ring.
Since equatorial hydrogens are positioned around the “equator” of the cyclohexane ring, they are relatively more stable than axial hydrogens. This stability arises from the fact that equatorial hydrogens experience less steric hindrance and minimal repulsion from other groups on the ring. In substituted cyclohexane derivatives, bulky substituents prefer the equatorial position to minimize unfavorable steric interactions, leading to a more stable conformation of the molecule.
Difference between Axial and Equatorial Hydrogen
The main differences between axial and equatorial hydrogens in cyclohexane are given in the following table:
Here is the comparison in table format:
| Feature | Axial Hydrogen | Equatorial Hydrogen |
|---|---|---|
| Orientation | Perpendicular to the ring plane (up/down) | Parallel to the ring plane (outward) |
| Position in Chair Conformation | Alternates up and down around the ring | Extends outward, roughly in the plane of the ring |
| Steric Hindrance | High (due to 1,3-diaxial interactions) | Low (less steric hindrance) |
| Stability | Less stable for bulky groups | More stable for bulky groups |
| Effect in Ring Flip | Becomes equatorial | Becomes axial |
| Preferred for Large Substituents | No (high steric strain) | Yes (more spatial freedom) |
In the chair conformation of cyclohexane, each carbon atom is attached to one axial hydrogen and two equatorial hydrogens. The distribution of axial and equatorial hydrogens contributes to the molecule’s stability and influences its physical and chemical properties.
Uses of Axial and Equatorial Positions (axial hydrogen and equatorial hydrogen) in Chemistry
The distinction between axial and equatorial positions in cyclic compounds, particularly cyclohexane, plays a crucial role in various chemical applications:
1. Stability and Conformational Analysis: Helps in predicting the most stable conformation of cyclohexane derivatives. Large substituents prefer the equatorial position to reduce steric hindrance, aiding in stability assessments.
2. Stereochemistry and Isomerism: Determines the spatial arrangement of substituents in cyclic molecules, influencing cis-trans isomerism. Used in designing chiral molecules for pharmaceutical and synthetic applications.
3. Drug Design and Medicinal Chemistry: Understanding axial vs. equatorial positioning helps in designing more bioavailable and stable drug molecules. Many steroid-based drugs rely on axial/equatorial conformations for receptor binding and biological activity.
4. Reaction Mechanisms and Selectivity: In nucleophilic substitution (SN1/SN2) and elimination (E1/E2) reactions, the axial or equatorial position affects reactivity.
Example: E2 eliminations favor β-hydrogens in the axial position due to the anti-periplanar requirement.
5. Organic Synthesis and Catalysis: Used in designing reaction pathways for cyclohexane-based compounds. Plays a role in the Diels-Alder reaction and other cycloaddition reactions.
6. Structural Analysis using Spectroscopy: Helps in NMR analysis of cyclic compounds, where axial and equatorial protons show different chemical shifts.
Understanding axial and equatorial positioning is essential in organic chemistry, pharmaceuticals, and molecular modeling for designing more effective and stable compounds.
Visit to: Pharmacareerinsider.com and Pharmaacademias.com




