Buffers and Their Importance
Buffers are solutions that resist changes in pH when an acid or base is added. They are crucial in various scientific, industrial, and biological processes, maintaining stable pH conditions. The ability of buffers to resist pH changes is attributed to a weak acid and its conjugate base (or a weak base and its conjugate acid).
Buffer Equation
The Henderson-Hasselbalch equation is a fundamental tool for understanding the behavior of buffers. It is expressed as:
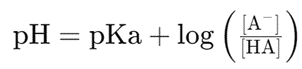
Where:
– pH is the measure of acidity or basicity.
– pKa is the negative logarithm (base 10) of the acid dissociation constant (Ka).
– A– is the concentration of the conjugate base.
– HA is the concentration of the weak acid.
Applications of Buffers
1. Biological Systems:
– Blood Buffering: The bicarbonate ion (HCO3-) system in the blood maintains the pH of blood around 7.4, which is crucial for enzyme activity and overall physiological function.
– Cellular pH Regulation: Intracellular buffers regulate the pH within cells, ensuring optimal conditions for biochemical processes.
2. Laboratory Techniques:
– Enzyme Assays: Buffers stabilize enzyme activity by maintaining the pH within a specific range.
– Polymerase Chain Reaction (PCR): Buffers in PCR reactions stabilize the pH, facilitating the amplification of DNA.
3. Chemical Reactions:
– Catalysis: Buffers are essential in catalytic processes, protecting catalysts from pH extremes.
– Hydrolysis Reactions: Buffers control pH during hydrolysis reactions, preventing rapid and undesirable changes.
4. Industrial Processes:
– Pharmaceuticals: Buffer solutions formulate medications to ensure stability and bioavailability.
– Food Processing: Buffers control pH in various food products, preserving taste, texture, and color.
5. Environmental Monitoring:
– Water Treatment: Buffers help maintain the pH of water, preventing damage to aquatic ecosystems and infrastructure.
– Soil Health: Buffers in soils resist changes in pH, influencing nutrient availability and plant growth.
Buffer Capacity
Buffer capacity refers to the ability of a buffer solution to resist changes in pH upon adding an acid or base. It is influenced by the concentrations of the weak acid and its conjugate base. Key aspects of buffer capacity include:
1. Buffer Range:
– Buffers are most effective within a specific pH range centered around the pKa of the weak acid.
2. Buffer Capacity Factors:
– Concentration: Higher concentrations of the weak acid and its conjugate base result in greater buffer capacity.
– pH Proximity to pKa: Buffer capacity is highest when pH is close to the pKa of the weak acid.
3. Optimizing Buffer Capacity:
– Choosing Buffer Components: Select appropriate weak acid-conjugate base pairs for the desired pH range.
– Adjusting Concentrations: Modifying concentrations of buffer components to enhance buffer capacity.
Buffers and their associated equations and capacities are indispensable in numerous fields. Their ability to maintain stable pH conditions is essential for the success of various biological, chemical, and industrial processes. Understanding the principles of buffers and their applications is vital for scientists, researchers, and professionals across diverse disciplines.

