Prolonged deprivation of oxygenated blood supply leads to irreversible cellular damage and dysfunction. The process of reversible cell injury can progress to irreversible cell injury, resulting in cell death. This irreversible state is marked by the cell’s inability to reverse mitochondrial and plasma membrane dysfunction even upon reperfusion or reoxygenation. Additional factors contributing to irreversible damage include sustained ATP depletion, decreased intracellular pH, and leakage of lysosomal enzymes into the cytoplasm. These biochemical alterations disrupt normal cell functions as follows:
1. Mitochondrial Damage
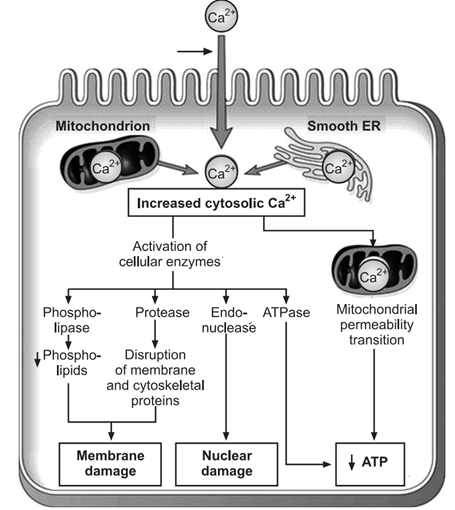
With continued oxygen deprivation, mitochondrial damage becomes irreversible. Reperfusion of the injured cell results in excess intracellular calcium accumulating in the mitochondria, disabling its function. Morphological changes include the formation of vacuoles and deposition of amorphous calcium salts in the mitochondrial matrix.
2. Membrane Damage
The most critical event in irreversible cell injury is plasma membrane damage, which impairs its normal function. This damage leads to increased cytosolic calcium influx, activating endogenous phospholipases. These enzymes degrade membrane phospholipids, essential components of the lipid bilayer. Additionally, activated ATPase enzymes further deplete ATP, reducing the synthesis of new phospholipids for membrane repair.
3. Cytoskeletal Damage
Intracellular proteases activated by increased calcium or the physical effects of cell swelling can damage the cytoskeleton, leading to further irreversible cell membrane injury.
4. Nuclear Damage
Lysosomal enzymes, such as proteases and endonucleases, damage nucleoproteins, resulting in irreversible nuclear damage, which can manifest in three forms:
Pyknosis: Condensation and clumping of the nucleus, appearing dark basophilic.
Karyorrhexis: Fragmentation of the nucleus into small pieces dispersed in the cytoplasm.
Karyolysis: Dissolution of the nucleus.
5. Lysosomal Damage, Cell Death, and Phagocytosis
Damage to lysosomal membranes leads to the release of hydrolytic enzymes into the cytoplasm. These enzymes, activated by low oxygen levels and acidic pH, include hydrolases, proteases, glycosidases, phosphatases, lipases, amylases, RNAases, and DNAases. Their activation results in the enzymatic digestion of cellular components, leading to cell death. Dead cells are replaced by phospholipid masses called myelin figures, which are either phagocytosed by macrophages or form calcium soaps. The release of these enzymes into the serum can serve as clinical markers of cell death. For instance, in myocardial infarction, elevated levels of SGPT, LDH, CKMB, and cardiac troponins indicate heart muscle cell death.
Free Radical Induced Injury
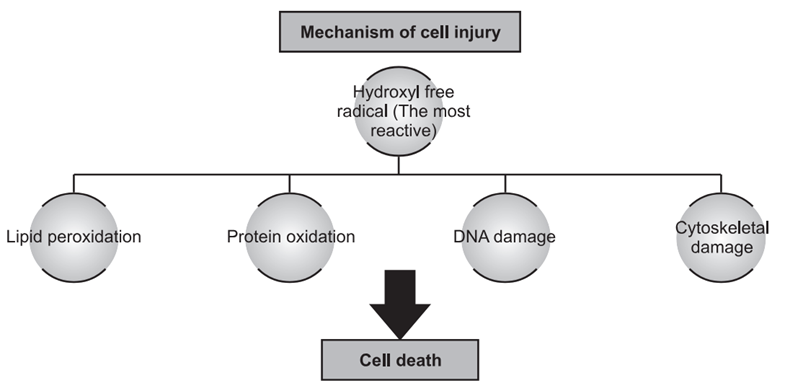
Free radicals, which have a single unpaired electron, cause cell damage by directly affecting cell membranes and initiating lethal sequences of events, particularly during reperfusion of ischemic cells. These activated oxygen radicals are a common cause of cell injury in conditions such as aging, chemical and radiation exposure, bacterial infections, inflammation, and tumor necrosis. Destructive free radicals, including superoxide radicals, hydroxyl ions, and peroxide ions, lead to lipid peroxidation, protein oxidation, DNA damage, and cytoskeletal damage. They are generated by both enzymatic and non-enzymatic reactions within cells.
Cells have protective mechanisms against free radicals, such as antioxidant enzymes like catalase, glutathione peroxidase, and superoxide dismutase. Additionally, vitamin E and selenium help protect cells from free radical-induced damage. Deficiencies in these protective mechanisms can result in significant cellular damage.
The deficiency of all these protective mechanism may lead to free radical reactive cellular damage, especially in muscle.
Causes of Free Radical Initiation
1. Ionizing Radiation: Ionizing radiation generates various free radical species by splitting molecules, producing free radical products.
2. Enzymatic Metabolism of Chemicals or Drugs: For example, carbon tetrachloride can generate the CCl₃ radical, leading to the autooxidation of polyenic fatty acids within membrane phospholipids.
3. Cellular Respiration: The electron transport chain in cellular respiration involves the regulated transfer of free radicals. Although tightly controlled, some free radicals can escape, especially when mitochondria are injured during metabolic cell injury.
4. Chemical Cell Injury: Metabolism of several exogenous chemicals can generate free radicals. Metals such as copper (Cu) and iron (Fe) participate in reactions (e.g., the Fenton reaction) that produce free radicals. Nitric oxide (NO) can act as a free radical and convert into highly reactive species like peroxynitrite (ONOO⁻), NO₂, and NO₃⁻. Normally, NO is produced by endothelial cells, neurons, and macrophages.
Free Radical Reactions in Normal Metabolism
During normal metabolic redox reactions, such as in respiration, molecular oxygen is reduced to water by accepting four electrons, producing small amounts of toxic intermediates. Free radical reactions include:
1. Lipid Peroxidation: Free radicals repeatedly attack polyunsaturated fatty acids (PUFAs) in cell membranes, forming highly destructive PUFA radicals like lipid hydroperoxy radicals and lipid hypoperoxides. This process, known as lipid peroxidation, spreads to adjoining parts of the membrane, causing extensive damage.
2. Protein Oxidation: Free radicals cause the oxidation and cleavage of protein macromolecules, leading to cross-linking of amino acids and fragmentation of polypeptides.
3. DNA Damage: Free radicals break DNA strands, resulting in defective DNA that cannot replicate, potentially leading to cell death.
4. Cytoskeleton Damage: Free radicals interfere with mitochondrial aerobic phosphorylation, decreasing ATP synthesis and causing cytoskeleton damage.
Endogenous antioxidants like Vitamin E, sulfhydryl-containing substances (e.g., cysteine), superoxide dismutase (SOD), catalase, glutathione (GTH), and serum proteins help combat oxidative damage from free radicals.




