The Electron Transport Chain (ETC) is a series of protein complexes and other molecules embedded in the inner mitochondrial membrane that plays a critical role in cellular respiration. It is the final stage of aerobic respiration, where the energy stored in NADH and FADH2, generated during glycolysis, the citric acid cycle, and beta-oxidation, is used to produce ATP. Here is a detailed examination of the ETC and its mechanism:
Structure of the Electron Transport Chain
The ETC consists of four main protein complexes (I-IV) and two mobile electron carriers: ubiquinone (coenzyme Q) and cytochrome c. These components are embedded in the inner mitochondrial membrane in eukaryotes (in the plasma membrane of prokaryotes).
1. Complex I (NADH: Ubiquinone Oxidoreductase):
– Also known as NADH dehydrogenase.
– Accepts electrons from NADH, which are transferred to flavin mononucleotide (FMN) and then through a series of iron-sulfur (Fe-S) clusters to ubiquinone.
– Pumps protons (H+) from the mitochondrial matrix to the intermembrane space, contributing to the proton gradient.
2. Complex II (Succinate: Ubiquinone Oxidoreductase):
– Also known as succinate dehydrogenase, part of both the citric acid cycle and the ETC.
– Accepts electrons from succinate via FADH2, which are then transferred through Fe-S clusters to ubiquinone.
– Does not pump protons, but transfers electrons to ubiquinone.
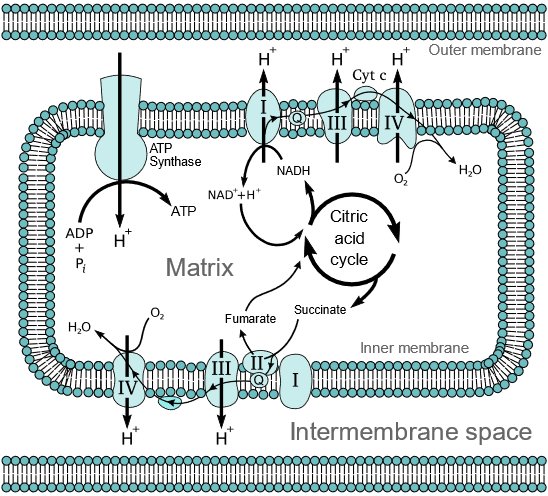
3. Ubiquinone (Coenzyme Q):
– A lipid-soluble molecule that shuttles electrons from Complex I and II to Complex III.
– Accepts electrons and protons, becoming reduced to ubiquinol (QH2).
4. Complex III (Ubiquinol: Cytochrome c Oxidoreductase):
– Also known as cytochrome bc1 complex.
– Transfers electrons from ubiquinol to cytochrome c via the Q-cycle, involving cytochromes b and c1 and the Rieske Fe-S protein.
– Pumps protons into the intermembrane space, further contributing to the proton gradient.
5. Cytochrome c:
– A small, water-soluble heme protein located in the intermembrane space.
– Shuttles electrons from Complex III to Complex IV.
6. Complex IV (Cytochrome c Oxidase):
– Accepts electrons from cytochrome c and transfers them to molecular oxygen (O2), reducing it to water (H2O).
– Contains heme groups and copper centers (CuA and CuB).
– Pumps protons into the intermembrane space.
Mechanism of the Electron Transport Chain
The ETC functions through a series of redox reactions where electrons are transferred from electron donors (NADH and FADH2) to electron acceptors (O2) via the complexes and mobile carriers. This process involves the following steps:
1. Electron Donation and Transfer:
– NADH donates electrons to Complex I, where they are transferred to FMN, then through Fe-S clusters to ubiquinone, reducing it to ubiquinol.
– FADH2 donates electrons to Complex II, where they are transferred through Fe-S clusters to ubiquinone, also reducing it to ubiquinol.
– Ubiquinol carries the electrons to Complex III.
2. Proton Pumping:
– As electrons pass through Complex I, III, and IV, energy released during these redox reactions is used to pump protons from the matrix to the intermembrane space.
1-Complex I pumps four protons per NADH.
2- Complex III pumps four protons per pair of electrons transferred through the Q-cycle.
3- Complex IV pumps two protons per pair of electrons.
3. Generation of Proton Motive Force (PMF):
– The pumping of protons into the intermembrane space creates an electrochemical gradient known as the proton motive force (PMF).
– This gradient consists of a difference in proton concentration (chemical gradient) and charge (electrical gradient) across the inner mitochondrial membrane.
4. ATP Synthesis:
– Protons flow back into the matrix through ATP synthase (Complex V), driven by the PMF.
– The flow of protons through ATP synthase provides the energy for the phosphorylation of ADP to ATP.
– This process is known as chemiosmosis.
5. Reduction of Oxygen:
– Electrons are transferred from cytochrome c to Complex IV, which then transfers them to molecular oxygen.
– Oxygen, the final electron acceptor, is reduced to water (H2O), a crucial step in maintaining the flow of electrons through the ETC.
Overall Reaction
The overall reaction of the ETC can be summarized as follows:
NADH + H++ 1/2 O2 → NAD+ + H2O
FADH2 + ½ O2→FAD + H2O
Importance of the Electron Transport Chain
1. ATP Production:
– The ETC is the main source of ATP in aerobic organisms. Each NADH can generate approximately 2.5 ATP molecules, and each FADH2 can generate approximately 1.5 ATP molecules through oxidative phosphorylation.
2. Heat Production:
– In some organisms, the proton gradient generated by the ETC is used to produce heat instead of ATP, a process known as non-shivering thermogenesis.
3. Reactive Oxygen Species (ROS):
– The ETC is also a source of reactive oxygen species (ROS), which are by-products of oxygen reduction. ROS can cause cellular damage but also play roles in cell signaling and homeostasis.
4. Cellular Metabolism:
– The ETC is integral to cellular respiration, linking glycolysis, the citric acid cycle, and beta-oxidation to ATP production. It is essential for energy metabolism in aerobic organisms.
Regulation of the Electron Transport Chain
The ETC is tightly regulated to meet the cell’s energy demands and to prevent excessive production of ROS. Key regulatory mechanisms include:
1. ADP Availability:
– ATP synthesis is tightly coupled to the availability of ADP. The rate of oxidative phosphorylation increases with ADP concentration.
2. NADH/FADH2 Levels:
– The availability of NADH and FADH2, produced by upstream metabolic pathways, regulates the flow of electrons through the ETC.
3. Oxygen Availability:
– Oxygen is the final electron acceptor. Low oxygen levels (hypoxia) can slow or halt the ETC, reducing ATP production.
4. Feedback Inhibition:
– High levels of ATP can inhibit components of the ETC, reducing electron flow and proton pumping.
5. Hormonal Regulation:
– Hormones such as insulin and glucagon can influence the activity of the ETC by modulating upstream metabolic pathways.
Summary
The Electron Transport Chain is a critical component of cellular respiration, facilitating the production of ATP through a series of redox reactions and proton pumping. It involves four main protein complexes and two mobile carriers, working together to create a proton gradient that drives ATP synthesis. The ETC is essential for energy metabolism, heat production, and cellular homeostasis, and is regulated to ensure efficient and balanced ATP production while minimizing oxidative stress.

