Labeling and packing of drugs and cosmetics are critical aspects regulated to ensure safety, efficacy, and proper use. The regulations vary by country but generally follow similar principles. Here’s a detailed note on the general labeling requirements and specimen labels for drugs and cosmetics:
General Labeling Requirements for Drugs
Labeling of pharmaceutical products is a critical aspect of regulatory compliance, ensuring drug safety, efficacy, and proper use. The requirements for drug labeling are governed by regulatory agencies such as the U.S. FDA (Food and Drug Administration), EMA (European Medicines Agency), CDSCO (Central Drugs Standard Control Organization – India), and WHO (World Health Organization).
1. Name of the Drug:
Brand Name: The proprietary name given by the manufacturer.
Generic Name: The official name of the drug.
2. Dosage Form and Strength: Indicates the form (tablet, capsule, injection, etc.) and the strength of the drug (e.g., 500 mg).
3. Route of Administration: Specifies how the drug should be taken (oral, intravenous, topical, etc.).
4. Batch Number: Unique identifier for a particular production batch, ensuring traceability.
5. Manufacturing and Expiry Dates: Dates indicating when the drug was manufactured and when it will expire.
6. Manufacturing License Number: License number issued by the regulatory authority, ensuring the legitimacy of the manufacturer.
7. Storage Conditions: Specific conditions under which the drug should be stored (e.g., store in a cool, dry place).
8. Warning and Precautionary Statements: Important safety information and potential side effects.
9. Directions for Use: Detailed instructions on how to use the drug properly.
10. Marketing Authorization Number: Number issued by the regulatory authority approving the drug for sale.
11. Net Content: Quantity of the drug in the package (e.g., number of tablets or volume of liquid).
12. Name and Address of Manufacturer: Complete address and name of the manufacturing entity.
13. Ingredients: Active and inactive ingredients, including their quantities.
General Labeling Requirements for Cosmetics
Cosmetic product labeling is essential for ensuring consumer safety, regulatory compliance, and proper product use. Labeling requirements vary across regulatory agencies such as the U.S. FDA (Food and Drug Administration), European Commission (EC Regulation No. 1223/2009), CDSCO (Central Drugs Standard Control Organization – India), and WHO (World Health Organization).
1. Product Name: The name by which the cosmetic product is known.
2. Net Quantity: The amount of product in the package (e.g., weight, volume).
3. Ingredients List: All ingredients listed in descending order of their concentration.
4. Batch Number: Identifier for a specific production batch.
5. Manufacturing and Expiry Dates: Indicates the dates of manufacturing and expiry.
6. Instructions for Use: Directions on how to use the product.
7. Warnings and Precautions: Safety information and potential side effects.
8. Country of Origin: Where the product was manufactured.
9. Manufacturer Details: Name and address of the manufacturer or distributor.
10. Certification Marks: Any applicable marks such as cruelty-free, organic, etc.
Specimen Labels for Drugs
Example 1: Oral Tablet
Front Label:
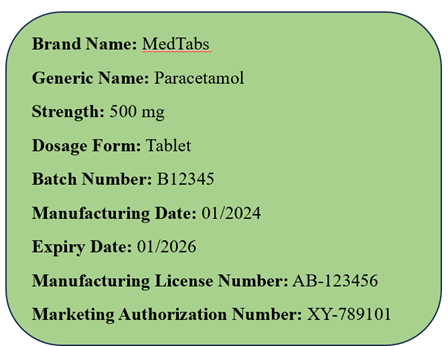
Back Label:

Example 2: Injectable Solution
Front Label:

Back Label:

Specimen Labels for Cosmetics
Example 1: Face Cream
Front Label:

Back Label:

Example 2: Shampoo
Front Label:

Back Label:

Regulatory References
Labeling requirements are typically guided by regulatory bodies such as:
– US FDA (Food and Drug Administration): Provides guidelines for drug and cosmetic labeling in the United States.
– EMA (European Medicines Agency): Regulates medicinal products within the European Union.
– TGA (Therapeutic Goods Administration): Oversees the labeling of therapeutic goods in Australia.
– MHRA (Medicines and Healthcare products Regulatory Agency): Regulates medicines, medical devices, and blood components for transfusion in the UK.
– CDSCO (Central Drugs Standard Control Organization): Regulates drugs and cosmetics in India.
Conclusion
Proper labeling and packing of drugs and cosmetics ensure consumer safety, facilitate informed usage, and enhance regulatory compliance. Manufacturers must adhere to these guidelines meticulously to maintain the trust and safety of consumers.

