The Shikimic Acid Pathway is an essential metabolic route in plants, fungi, and some bacteria, leading to the synthesis of aromatic amino acids and a diverse range of secondary metabolites. This pathway plays a central role in the metabolism of these organisms, connecting carbohydrate metabolism with the biosynthesis of phenylpropanoids, alkaloids, and other aromatic compounds. Because the pathway is absent in animals, it represents an attractive target for antimicrobial drugs, herbicides, and other agricultural applications.
Detailed Overview of the Shikimic Acid Pathway
The Shikimic acid pathway is a seven-step metabolic route starting from simple sugars and culminating in the production of chorismate, a precursor for the synthesis of various aromatic compounds. The pathway can be divided into three main phases:
1. Prechorismate Phase: Begins with the conversion of phosphoenolpyruvate (PEP) and erythrose 4-phosphate (E4P) into 3-Deoxy-D-arabino-heptulosonate-7-phosphate (DAHP). This is followed by a series of steps leading to the production of shikimic acid.
2. Conversion to Chorismate: Shikimic acid undergoes phosphorylation and further reactions to form 5-enolpyruvylshikimate-3-phosphate (EPSP) and, finally, chorismate.
3. Postchorismate Phase: Chorismate serves as a branching point for multiple biosynthetic pathways, leading to various secondary metabolites like aromatic amino acids (phenylalanine, tyrosine, and tryptophan), folates, ubiquinones, and others.
Step-by-Step Breakdown of the Shikimic Acid Pathway
1. Formation of DAHP (3-Deoxy-D-arabino-heptulosonate-7-phosphate):
Enzyme: DAHP Synthase
Reaction: Condensation of PEP (from glycolysis) and E4P (from the pentose phosphate pathway) catalyzed by DAHP synthase.
Significance: This is the initial step that links the central carbohydrate metabolism to aromatic compound biosynthesis.
2. Conversion of DAHP to 3-Dehydroquinate (DHQ):
Enzyme: DAHP Dehydratase
Reaction: Cyclization and reduction of DAHP to produce 3-Dehydroquinate.
Intermediate Products: This step forms a cyclic intermediate that will be further reduced.
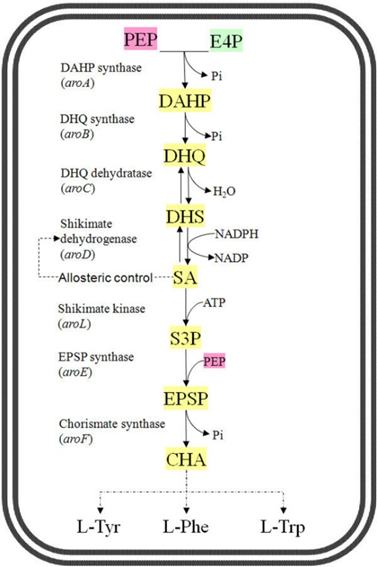
3. Reduction of DHQ to 3-Dehydroshikimate (DHS):
Enzyme: 3-Dehydroquinate Dehydratase
Reaction: Dehydration of DHQ to produce 3-dehydroshikimate.
Significance: Removal of a water molecule is crucial for the subsequent reduction step.
4. Formation of Shikimic Acid:
Enzyme: Shikimate Dehydrogenase
Reaction: Reduction of 3-Dehydroshikimate to Shikimic Acid.
Cofactor: NADPH acts as a reducing agent.
Importance: Shikimic acid is a pivotal intermediate for further reactions in the pathway.
5. Phosphorylation to Form Shikimate-3-Phosphate:
Enzyme: Shikimate Kinase
Reaction: Phosphorylation of shikimic acid to produce shikimate-3-phosphate.
ATP Utilization: This step requires ATP, emphasizing its energy dependence.
6. Formation of 5-Enolpyruvylshikimate-3-Phosphate (EPSP):
Enzyme: EPSP Synthase
Reaction: Condensation of shikimate-3-phosphate with another molecule of PEP.
Significance: This step is targeted by glyphosate, a common herbicide, as inhibition of EPSP synthase blocks the entire pathway.
7. Formation of Chorismate:
Enzyme: Chorismate Synthase
Reaction: Cyclization of EPSP to form chorismate.
Outcome: Chorismate is a crucial branching point leading to multiple secondary metabolic pathways.
Secondary Metabolite Formation via the Shikimic Acid Pathway
1. Aromatic Amino Acids:
Phenylalanine and Tyrosine:
Pathway: Chorismate is converted to prephenate, which undergoes separate reactions to form phenylalanine and tyrosine.
Enzymes: Prephenate dehydratase (for phenylalanine) and prephenate dehydrogenase (for tyrosine).
Role: Precursors for flavonoids, lignins, and other phenolic compounds.
Tryptophan:
Pathway: Chorismate is converted to anthranilate, which undergoes multiple reactions to produce tryptophan.
Enzymes: Anthranilate synthase, indole-3-glycerol phosphate synthase, and tryptophan synthase.
Role: A precursor for many indole alkaloids.
2. Phenylpropanoids:
Formation: Derived from phenylalanine via the phenylpropanoid pathway.
Key Metabolites: Flavonoids, coumarins, stilbenes, and lignins.
Importance: Provide structural components like lignin, UV protection, pigmentation, and defense compounds.
3. Flavonoids and Anthocyanins:
Pathway: Utilizes phenylalanine through a series of enzymatic transformations involving chalcone synthase and other enzymes.
Function: Responsible for flower pigmentation, protection against UV radiation, and antimicrobial activities.
4. Alkaloids:
Indole Alkaloids:
Precursor: Derived from tryptophan, leading to compounds such as vinblastine and vincristine (anticancer drugs).
Role: These compounds have various pharmacological activities, including anticancer, analgesic, and antimalarial properties.
5. Tannins:
Pathway: Produced via the condensation of flavan-3-ols or gallic acid derivatives.
Function: Serve as plant defense compounds against herbivores and pathogens, and are involved in reducing oxidative stress.
6. Lignins:
Formation: Derived from phenylalanine through a series of oxidation and polymerization reactions.
Importance: Provide rigidity and structural support to plant cell walls and are crucial for water transport.
7. Vitamins:
Folates and Ubiquinones:
Pathway: Chorismate is a precursor for p-aminobenzoic acid (PABA), which is essential for folate synthesis.
Role: These compounds are crucial for DNA synthesis and electron transport chain functions.
Conclusion
The Shikimic acid pathway is fundamental to the production of aromatic amino acids and a vast array of secondary metabolites. These compounds are essential for the survival, growth, and adaptation of plants and microbes, providing structural components, defense mechanisms, and bioactive molecules with pharmacological properties. Understanding the intricacies of this pathway opens avenues for genetic engineering, drug development, and agricultural innovation.


 Join Our Telegram Channel
Join Our Telegram Channel