Thin Layer Chromatography (TLC) is a widely used analytical technique that separates and identifies compounds in a mixture. It is based on the differences in the migration rate of compounds when carried by a mobile phase (solvent) over a stationary phase (adsorbent layer).
Thin Layer Chromatography (TLC) is a type of planar chromatography. It is used to analyze mixtures qualitatively and sometimes quantitatively. The stationary phase in TLC is a thin layer of adsorbent material like silica gel, alumina, or cellulose coated onto a solid support (e.g., glass, plastic, or aluminum plate).
TLC is simple, fast, and economical, making it popular in chemical and pharmaceutical laboratories for the following purposes:
– Identifying compounds.
– Testing the purity of substances.
– Monitoring the progress of chemical reactions.
Principle of Thin Layer Chromatography
The separation in TLC occurs due to the interaction of the components of a mixture with two phases:
1. Stationary Phase: A polar adsorbent material (e.g., silica gel) that interacts with the compounds primarily through adsorption.
2. Mobile Phase: A liquid solvent or solvent mixture that carries the sample up the plate through capillary action.
When a sample is spotted onto the plate and the plate is placed in the mobile phase, components move at different rates depending on their:
– Adsorption Affinity: Components strongly adsorbed onto the stationary phase travel slowly.
– Solubility in Mobile Phase: Components highly soluble in the mobile phase travel faster.
This differential migration causes the separation of components into distinct spots.
Methodology
1. Preparation of the TLC Plate
– The stationary phase is applied as a thin, uniform layer (0.1–0.3 mm thick) on a solid support.
– Plates are pre-coated commercially or prepared in the lab.
– The plate is activated by heating at 100–120°C for 30 minutes to remove moisture.
2. Sample Application
– A small volume of the sample solution (1–10 μL) is applied as a small spot near the bottom edge of the plate (baseline), usually 1–2 cm from the edge.
– Spotting is done using a capillary tube or micropipette. The spots should be small to ensure better separation.
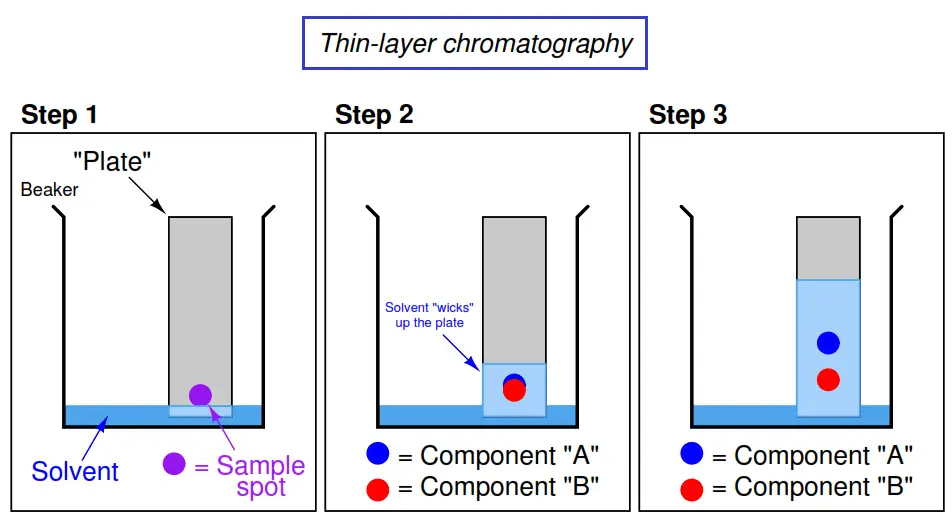
3. Development of the Chromatogram
– The plate is placed vertically in a development chamber containing the mobile phase.
– The chamber is pre-saturated with the mobile phase vapors to ensure even migration.
– The mobile phase ascends the plate by capillary action, carrying the sample components at varying rates.
4. Detection of Spots
– Once the solvent front reaches a desired height (5–10 cm), the plate is removed and dried.
– Spots are visualized under:
– UV Light: Common for compounds that fluoresce or absorb UV.
– Iodine Vapors: For organic compounds like hydrocarbons.
– Chemical Sprays: Such as ninhydrin for amino acids or Dragendorff’s reagent for alkaloids.
5. Calculation of Rf Values
The Retention Factor (Rf) is calculated to identify the separated components:
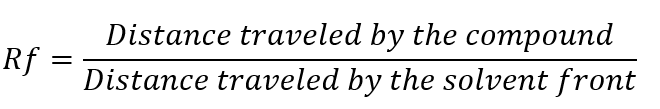
– Rf values are specific for a given compound under particular experimental conditions.
Retention Factor (Rf) Values
The Rf value is a crucial parameter for identifying compounds.
– Range: Rf values range from 0 (no migration) to 1 (travels with the solvent front).
Factors Affecting Rf Values:
– Nature of the stationary phase.
– Composition of the mobile phase.
– Temperature and humidity.
Example: In a mixture, a compound traveling 4 cm while the solvent front moves 10 cm has an Rf value of:

Advantages of TLC
1. Ease of Use: No sophisticated instruments required.
2. Cost-Effective: Inexpensive materials and setup.
3. Rapid Results: Chromatography is completed within minutes to hours.
4. Versatile Detection: Various visualization techniques can detect colorless compounds.
5. Low Sample Requirement: Requires only micrograms of the sample.
6. Simultaneous Analysis: Multiple samples can be run on a single plate.
Disadvantages of TLC
1. Limited Sensitivity: Not suitable for detecting very low concentrations.
2. Poor Resolution: Overlapping spots can occur with complex mixtures.
3. Non-Reproducibility: Results vary with plate preparation, solvent composition, and environmental conditions.
4. Semi-Quantitative: TLC primarily offers qualitative information.
Applications of TLC
1. Pharmaceutical Industry
– Analyzing drug formulations.
– Detecting impurities and degradation products.
– Identifying active pharmaceutical ingredients (APIs).
2. Chemical Research
– Monitoring chemical reactions.
– Identifying organic compounds.
3. Biochemical Analysis
– Analyzing amino acids, proteins, and lipids.
– Detecting nucleotides and sugars.
4. Forensic Science
– Identifying drugs, toxins, and inks in crime investigations.
5. Food Industry
– Detecting adulterants, preservatives, and dyes.
– Analyzing natural products like essential oils.
6. Environmental Studies
– Identifying pollutants in water, air, and soil samples.
Thin Layer Chromatography (TLC) is an essential technique for the qualitative and semi-quantitative analysis of compounds. Its simplicity, affordability, and adaptability make it a cornerstone in many scientific fields. However, its limitations in sensitivity and reproducibility should be considered when interpreting results.




