Acid-base titrations are a category of chemical titrations that involve the neutralization of an acid with a base or vice versa. These titrations are classified based on the nature of the reactants and the type of analysis being performed. Here’s a note on the main classifications of acid-base titrations:
Theory of Titration for Strong, Weak, and Very Weak Acids and Bases
Titration is an analytical technique used to determine the concentration of an unknown acid or base by reacting it with a standard solution of known concentration. The behavior of acids and bases in titration depends on their strength, which is determined by their dissociation in water. The pH changes during titration vary depending on whether the acid and base involved are strong, weak, or very weak.
1. Titration of Strong Acids and Strong Bases
Titration between a strong acid and a strong base is one of the most common types of acid-base titrations. Since both the acid and the base completely dissociate in aqueous solutions, the reaction proceeds to completion, resulting in a sharp and well-defined equivalence point.
1. Theory of Strong Acid-Strong Base Titration
In a strong acid-strong base titration, a strong acid (e.g., HCl) is titrated with a strong base (e.g., NaOH). The reaction follows the neutralization reaction:
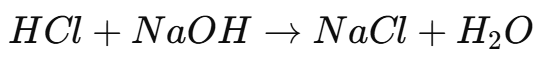
Strong acid: Completely ionizes in water (e.g., HCl → H⁺ + Cl⁻).
Strong base: Completely ionizes in water (e.g., NaOH → Na⁺ + OH⁻).
Equivalence point: pH = 7 because only water and a neutral salt (NaCl) are formed.
pH change: Rapid and steep near the equivalence point, making it easy to detect.
2. pH Changes During Titration
(a) Initial pH (Before Adding NaOH)
The solution contains only HCl, which is a strong acid.
The pH can be calculated using:
pH=−log[H+]
For 0.1 M HCl:
pH=−log(0.1)=1
(b) Before Equivalence Point (Excess HCl Present)
Since NaOH is being added, some H⁺ ions react with OH⁻ to form water.
However, as long as HCl is in excess, the solution remains acidic.
The pH can still be calculated using:
pH=−log[H+]
where [H⁺] is the remaining concentration of HCl after partial neutralization.
(c) At Equivalence Point
Equal moles of HCl and NaOH have reacted, leaving only neutral NaCl and water.
pH = 7, because neither Na⁺ nor Cl⁻ hydrolyzes in water.
(d) After Equivalence Point (Excess NaOH Present)
Any additional NaOH added results in excess OH⁻ ions.
The pH can be calculated using:
pOH=−log[OH−],pH=14−pOH
For 0.01 M excess NaOH:
pOH=−log(0.01)=2,pH=14−2=12
2. Titration of Weak Acids and Strong Bases:
Weak acid-strong base titration
In weak acid-strong base titrations, the analyte is a weak acid (partially ionized) and the titrant is a strong base. An example is acetic acid (CH₃COOH) titration with sodium hydroxide (NaOH).
Theory
The titration involves gradually adding a strong base to a weak acid solution. The weak acid partially ionizes to release H⁺ ions and reacts with the strong base.
At the equivalence point, all the weak acid is converted to its conjugate base, and the solution is basic (pH > 7).
Example Reaction: CH₃COOH + NaOH → CH₃COONa + H₂O
The pH at the equivalence point depends on the weak acid’s dissociation constant (Ka).
3. Titration of Very Weak Acids and Strong Bases
Very weak acid-strong base titration
In these titrations, the analyte is a fragile acid, such as ammonia (NH₄OH), and the titrant is a strong base.
Theory
The titration of very weak acids with strong bases is unique because the reaction is more complex. The analyte (NH₄OH) acts as a weak acid, but it can also behave as a weak base.
The primary reaction involves the reaction of NH₄OH with OH⁻ ions to form NH₄⁺ ions and water. However, NH₄⁺ ions can also react with OH⁻ ions to form NH₃ and water.
The relative concentrations of NH₄⁺ and NH₃ determine the pH at the equivalence point. If NH₄⁺ is in excess, the solution is acidic (pH < 7), while an excess of NH₃ makes the solution basic (pH > 7).
Example Reaction: NH₄OH + NaOH → NH₄⁺ + OH⁻ + Na⁺ + H₂O
4. Titration of Weak Bases and Strong Acids
Weak base-strong acid titration
The analyte is a weak base in these titrations, and the titrant is a strong acid. An example is ammonia (NH₃) titration with hydrochloric acid (HCl).
Theory
The titration involves gradually adding a strong acid to a weak base solution. The weak base partially ionizes to release OH⁻ ions and reacts with the strong acid.
At the equivalence point, all the weak base is converted to its conjugate acid, and the solution is acidic (pH < 7).
Example Reaction: NH₃ + HCl → NH₄⁺ + Cl⁻
The pH at the equivalence point depends on the weak base’s dissociation constant (Kb).
Visit to: Pharmacareerinsider.com and Pharmaacademias.com


2 thoughts on “Classification of Acid-Base Titrations”