Acidifiers are substances or compounds that can increase the acidity of a solution or a substance. Various industries, including food and agriculture, commonly use acidifiers to adjust the pH levels of products. In the food industry, acidifiers are added to enhance flavor, act as preservatives, and provide a desirable acidic taste. In agriculture, they play a role in modifying the pH of soil or water to create optimal conditions for plant growth. “acidifier” is broad and can refer to different substances, including organic, inorganic, or acidic salts.
Ammonium Chloride as Acidifier
Ammonium chloride (NH₄Cl) functions as an acidifier in various applications, leveraging its acidic properties derived from the combination of ammonia and hydrochloric acid. Various industries employ ammonium chloride to regulate pH in different applications.
Assay of Ammonium chloride
An assay is a quantitative analysis method used to determine the concentration or purity of a substance. A common assay method for ammonium chloride involves titration with a base, such as sodium hydroxide (NaOH). Here’s a simplified outline of the assay procedure:
Assay of Ammonium Chloride by Titration
Materials
1. Ammonium chloride sample
2. Sodium hydroxide (NaOH) solution of known concentration (standard solution)
3. Indicator (phenolphthalein is commonly used)
4. Distilled water
5. Burette
6. Conical flask
Procedure
1. Sample Preparation:
Weigh a known amount of ammonium chloride sample accurately.
2. Titration
Dissolve the ammonium chloride sample in distilled water in a conical flask.
Add a few drops of the chosen indicator (e.g., phenolphthalein) to the solution.
Titrate the ammonium chloride solution with the sodium hydroxide solution from the burette.
Carry out the titration until you observe a color change in the solution, indicating the endpoint of the reaction.
3. Reaction
The reaction involves the reaction between ammonium chloride and sodium hydroxide:
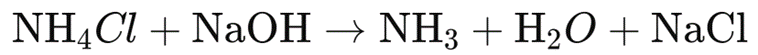
4. Calculations
Calculate the amount of ammonium chloride in the sample using the volume and concentration of the sodium hydroxide solution.
Note
The molarity of the sodium hydroxide solution is crucial for accurate calculations.
Use the balanced chemical equation to determine the stoichiometry of the reaction.
Precautions
Ensure accurate weighing and measuring of reagents.
Perform the titration carefully and slowly near the endpoint to achieve precision.
Use proper laboratory safety measures.
It’s important to note that specific procedure details may vary based on the laboratory’s requirements and the equipment available. Always refer to the standard operating procedures and guidelines provided by the laboratory or analytical method.
Properties of ammonium chloride
1. Chemical Structure: Ammonium chloride consists of positively charged ammonium ions (NH₄⁺) and negatively charged chloride ions (Cl⁻).
2. Acidic Nature: When dissolved in water, ammonium chloride dissociates, releasing hydrogen ions (H⁺) into the solution. This process contributes to its acidic properties.
Medicinal uses of Ammonium chloride
Ammonium chloride (NH₄Cl) has various medicinal uses, and it is employed in the pharmaceutical industry for its specific properties. Here are some medicinal uses of ammonium chloride:
1. Expectorant: Ammonium chloride is used as an expectorant in cough medicines. It helps thin and loosen mucus in the airways, making coughing and clearing respiratory passages easier.
2. Acidifying Agent: It is used as an acidifying agent in certain medications to maintain an acidic pH. This is particularly relevant in the formulation of urinary acidifiers.
3. Treatment of Alkalosis: Under medical supervision, ammonium chloride is sometimes administered to treat certain types of alkalosis, a condition characterized by excessively alkaline body fluids, in order to help restore a more balanced pH.
4. Diuretic Effect: Sometimes, medical professionals may use ammonium chloride as a diuretic to increase urine production. They carefully monitor its use due to its potential benefits in specific medical conditions.
5. Urinary Acidification: In certain clinical situations, such as treating urinary tract infections or preventing certain kidney stones, medical professionals may use ammonium chloride to acidify the urine.
6. Diagnostic Agent: In some diagnostic tests, including the Nessler’s reagent test for detecting ammonia in urine, professionals use ammonium chloride.
7. Intravenous Infusion: Under medical supervision, healthcare providers may administer ammonium chloride intravenously in certain medical situations.
It’s crucial to note that using ammonium chloride in medicinal applications should be under healthcare professionals’ guidance. Self-medication or misuse of ammonium chloride can lead to adverse effects, and its use should be based on a healthcare provider’s recommendation and prescription.
Considerations and Safety
1. Dosage Control: The amount of ammonium chloride must be carefully controlled, especially in food applications, to prevent excessive acidity.
2. Health and Safety: While ammonium chloride is generally considered safe in moderate amounts, excessive consumption may have health implications. It is essential to adhere to the recommended dosage levels.
3. Environmental Impact: The use of ammonium chloride in agriculture may contribute to soil acidification, and its environmental impact should be considered. Ammonium chloride is a versatile acidifier with applications in food, agriculture, and various industrial processes. Its controlled use allows for regulating pH levels, making it valuable in different contexts. However, proper dosage control and consideration of safety and environmental factors are crucial in its application.

