The Amino Acid Pathway is a critical metabolic route that not only contributes to protein synthesis but also serves as a precursor for a wide variety of secondary metabolites. These secondary metabolites, derived from amino acids, play significant roles in plant defense, pigmentation, aroma, flavor, and therapeutic applications. Amino acid-derived secondary metabolites include alkaloids, glucosinolates, cyanogenic glycosides, and many other bioactive compounds.
Overview of the Amino Acid Pathway
Amino acids are synthesized through different biosynthetic pathways depending on the organism. In plants, fungi, and microorganisms, these pathways converge to produce essential and non-essential amino acids. Each amino acid can undergo specific enzymatic transformations to yield a range of secondary metabolites. The pathway involves multiple enzymatic reactions like transamination, decarboxylation, oxidation, and condensation, leading to the formation of diverse structures
Key Amino Acids Involved in Secondary Metabolite Formation
1. Phenylalanine and Tyrosine:
Source: Derived from the shikimic acid pathway.
Key Enzyme: Phenylalanine ammonia-lyase (PAL) converts phenylalanine to cinnamic acid, a precursor for various phenylpropanoids and flavonoids.
Secondary Metabolites:
Alkaloids: Ephedrine, dopamine, and morphine.
Flavonoids: Anthocyanins, quercetin, and catechins.
Lignin: A complex polymer contributing to plant cell wall rigidity.
Coumarins: Umbelliferone and scopoletin.
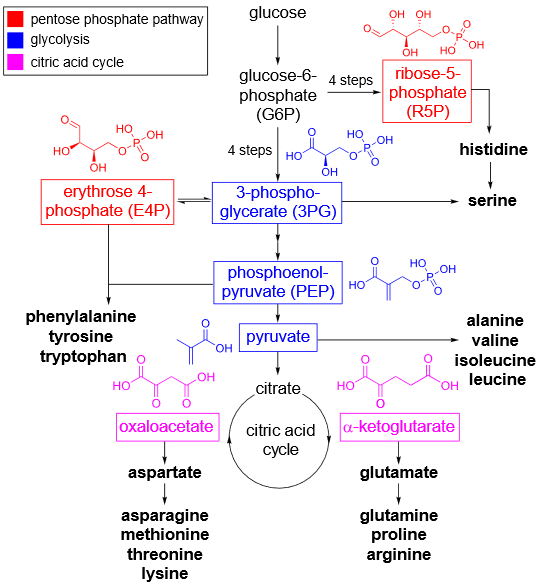
2. Tryptophan:
Source: Synthesized via the shikimic acid pathway.
Key Enzymes: Tryptophan decarboxylase and indole-3-glycerol phosphate synthase.
Secondary Metabolites:
Indole Alkaloids: Reserpine, strychnine, and ergot alkaloids.
Auxins: Indole-3-acetic acid (IAA), a plant growth regulator.
Glucosinolates: Compounds involved in plant defense, derived from indole-3-acetaldoxime.
3. Lysine:
Source: Derived from the aspartate pathway.
Key Enzyme: Diaminopimelate decarboxylase.
Secondary Metabolites:
Piperidine Alkaloids: Such as piperine, found in black pepper.
β-lactams: Such as clavulanic acid, an antibiotic compound.
4. Histidine:
Source: Synthesized from ribose 5-phosphate through the pentose phosphate pathway.
Secondary Metabolites:
Histamine: A mediator of allergic responses.
Benzylisoquinoline Alkaloids: Such as berberine and morphine, derived via histidine’s involvement in metabolic routes.
5. Ornithine and Arginine:
Source: Derived from glutamate.
Key Enzymes: Ornithine decarboxylase and arginine decarboxylase.
Secondary Metabolites:
Polyamines: Putrescine, spermidine, and spermine involved in cell growth and differentiation.
Pyrrolizidine Alkaloids: Toxic secondary metabolites used by plants for defense.
Nicotine: An alkaloid derived from ornithine and involved in plant defense mechanisms.
6. Cysteine:
Source: Synthesized from serine via sulfhydrylation.
Secondary Metabolites:
Glucosinolates: Sulfur-containing compounds found in Brassicaceae family plants.
Alliins: Found in garlic and onion, responsible for characteristic aromas and health benefits.
Formation of Secondary Metabolites through Amino Acid Pathways
1. Alkaloids:
Definition: Nitrogen-containing secondary metabolites with diverse pharmacological activities.
Biosynthesis: Derived from various amino acids like tyrosine, tryptophan, lysine, and ornithine.
Types and Examples:
Indole Alkaloids: Derived from tryptophan (e.g., vincristine, vinblastine).
Isoquinoline Alkaloids: Derived from tyrosine (e.g., codeine, morphine).
Piperidine Alkaloids: Derived from lysine (e.g., lobeline).
2. Phenylpropanoids and Flavonoids:
Definition: A large group of plant secondary metabolites derived from phenylalanine.
Biosynthesis: Phenylalanine is deaminated by PAL to form cinnamic acid, which undergoes various enzymatic modifications to produce different phenylpropanoids.
Types and Examples:
Flavonoids: Anthocyanins, flavonols, and isoflavones.
Stilbenes: Resveratrol.
Lignans and Lignins: Important for cell wall structure and defense.
3. Glucosinolates:
Definition: Sulfur-containing compounds derived from amino acids like tryptophan, phenylalanine, and methionine.
Biosynthesis: Amino acids undergo side-chain elongation, oxidation, and glycosylation.
Functions: Act as defense compounds against herbivores and pathogens; have potential health benefits in humans.
4. Cyanogenic Glycosides:
Definition: Toxic compounds that release hydrogen cyanide upon hydrolysis.
Biosynthesis: Derived from amino acids such as phenylalanine, tyrosine, and valine.
Examples: Linamarin, lotaustralin, and amygdalin.
5. Non-Protein Amino Acids:
Definition: Amino acids that are not incorporated into proteins but play roles in plant defense and stress response.
Biosynthesis: Synthesized directly from proteinogenic amino acids.
Examples: Azetidine-2-carboxylic acid and canavanine.
Conclusion
The amino acid pathway is integral to the biosynthesis of numerous secondary metabolites that play essential roles in plant defense, human health, and ecological interactions. The diversity of structures and functions of these metabolites highlights the metabolic versatility of organisms in utilizing amino acids beyond their role in protein synthesis.

