Analytical evidence in the derivation of the structure of benzene
Analytical evidence played a crucial role in elucidating the structure of benzene, contributing to the understanding of its unique stability and aromatic properties. The analytical methods provided experimental data supporting the theoretical models proposed for benzene’s structure. Here’s a detailed look at the analytical evidence in the derivation of the structure of benzene:
1. Empirical Formula Determination:
Early analyses revealed that the empirical formula of benzene is C6H6, suggesting a high degree of unsaturation. The observation of a 1:1 carbon-to-hydrogen ratio in combustion reactions provided initial evidence.
2. Molecular Weight Measurements:
Cryoscopic and ebullioscopic methods were used to determine the molecular weight of benzene. The calculated molecular weight was consistent with the empirical formula, pointing towards a molecular formula of C6H6. This supported the notion that benzene had an unusual structure with fewer hydrogen atoms than expected for a saturated hydrocarbon.
3. Combustion Analysis:
Combustion analysis further confirmed the empirical formula by determining benzene’s carbon and hydrogen content. The results supported the 1:1 carbon-to-hydrogen ratio, reinforcing that benzene had a distinctive structure.
4. X-ray Crystallography:
X-ray crystallography studies provided direct visualization of the arrangement of atoms in benzene molecules. The crystal structure analysis demonstrated a planar hexagonal arrangement of carbon atoms, supporting benzene’s cyclic and conjugated nature.
5. Magnetic Susceptibility Measurements:
Measurements of magnetic susceptibility provided information about the electronic structure of benzene. The observed magnetic properties were consistent with a cyclic, conjugated system of electrons, reinforcing the aromatic character of benzene.
6. Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR spectroscopy experiments confirmed the equivalent nature of all hydrogen atoms in benzene. The single peak in the NMR spectrum for benzene’s hydrogen atoms supported a symmetric, planar structure consistent with the resonance hybrid model.
7. Isotopic Substitution:
Isotopic substitution experiments involved replacing hydrogen atoms in benzene with deuterium. The uniform electron density distribution observed in these experiments supported the idea of delocalized pi electrons and reinforced the resonance structure concept.
Analytical methods collectively provided valuable experimental data supporting the benzene structural models. The empirical formula determination, molecular weight measurements, and spectroscopic techniques such as X-ray crystallography and NMR spectroscopy played pivotal roles in confirming benzene’s cyclic, planar, and aromatic nature. This analytical evidence was essential in validating the theoretical frameworks, leading to the acceptance of the resonance hybrid model for benzene’s structure.
Synthetic Evidence in the Derivation of the Structure of Benzene
The determination of the structure of benzene involved a significant contribution from synthetic evidence. Synthetic studies, including reactions and manipulations of benzene and its derivatives, provided critical insights into the reactivity and behavior, supporting the development of the resonance hybrid model. Here is a detailed examination of synthetic evidence in the derivation of the structure of benzene:
1. Electrophilic Aromatic Substitution Reactions
Reaction Description: Benzene exhibits a unique reactivity in electrophilic aromatic substitution reactions. For instance, benzene undergoes substitution rather than addition reactions in the presence of bromine and a Lewis acid catalyst like FeBr3.
Significance: The observed electrophilic substitution supported the idea of a stable, aromatic compound. The resistance of benzene to typical alkene reactions contradicted the structure of alternating single and double bonds and prompted the exploration of alternative models.
2. Friedel-Crafts Reactions
Reaction Description: The Friedel-Crafts alkylation and acylation reactions involve introducing alkyl or acyl groups onto the benzene ring in the presence of a Lewis acid catalyst, such as AlCl3.
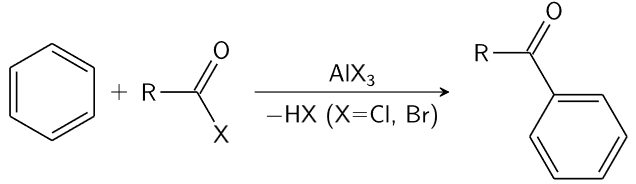

Significance: These reactions demonstrated the ability of benzene to undergo substitution reactions. The fact that benzene was not readily saturated but could still undergo these substitution reactions supported the idea of a stable, unsaturated structure.
3. Nitration and Halogenation Reactions
Reaction Description: Benzene undergoes nitration (substitution of hydrogen with a nitro group) and halogenation (substitution of hydrogen with a halogen) reactions, demonstrating its reactivity toward electrophiles.
Significance: The observed substitution reactions were consistent with an aromatic system. The lack of addition reactions typical of alkenes suggested that the structure of benzene was distinct from alternating single and double bonds.
4. Hydrogenation Studies
Reaction Description: When subjected to hydrogenation (addition of hydrogen), Benzene does not readily undergo saturation reactions under typical conditions.
Significance: The lack of reactivity in hydrogenation reactions contradicted the expected behavior for a compound with alternating double bonds. The stability observed in these studies supported the proposal of a unique, stable structure.
5. Isolation of Benzene from Natural Sources
Procedure: Distillation can isolate Benzene from natural sources, such as crude oil.
Significance: The isolation of benzene from natural sources provided evidence of its existence and stability in its native environment.
6. Synthesis of Benzene Derivatives
Reaction Description: Benzene derivatives, such as toluene (methylbenzene), aniline (aminobenzene), and phenol (hydroxybenzene), are synthesized by introducing various substituents to the benzene ring.
Significance: The successful synthesis of derivatives highlighted the versatility and reactivity of benzene, supporting the proposed aromatic structure.
Synthetic evidence played a pivotal role in supporting the proposed structure of benzene. The unique reactivity patterns observed in electrophilic aromatic substitution, Friedel-Crafts reactions, and hydrogenation studies, coupled with the successful synthesis of benzene derivatives, provided strong support for benzene’s cyclic, planar, and aromatic nature. These synthetic investigations were essential in validating the resonance hybrid model and establishing the foundational principles of aromaticity in organic chemistry.
Other Evidence in the Derivation of the Structure of Benzene
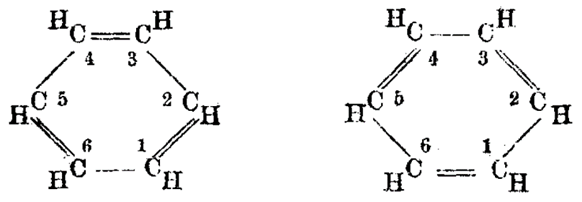
1. Kekulé’s Structural Hypothesis
Kekulé initially proposed a cyclic structure for benzene with alternating single and double bonds. While this model was inconsistent with observed properties, it sparked further investigation.
2. Resonance Theory
The resonance theory development explained benzene stability through the delocalization of pi electrons. This theory was essential in reconciling experimental observations with structural models.
3. Quantum Mechanics
Quantum mechanical calculations further supported the idea of a cyclic, delocalized pi-electron system, providing a theoretical foundation for the resonance hybrid model.
4. Hydrogenation Studies
Hydrogenation studies, specifically the reaction of benzene with hydrogen in the presence of a metal catalyst, demonstrated that benzene does not undergo typical saturation reactions, supporting a stable, unsaturated structure.
The determination of the structure of benzene involved a comprehensive approach, combining analytical methods, synthetic reactions, and theoretical considerations. The culmination of this evidence led to the acceptance of the resonance hybrid model, explaining benzene’s unique stability and aromatic properties.

