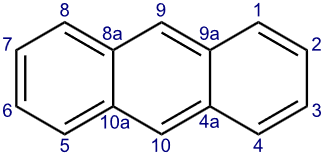
Structure of Anthracene
Anthracene is a tricyclic aromatic hydrocarbon consisting of three fused benzene rings. Its molecular formula is C14H10, and the structure comprises three six-membered rings arranged linearly. Anthracene is planar and exhibits aromaticity due to the delocalized π electrons in the benzene rings.
Medicinal Uses of Anthracene
Anthracene, a polycyclic aromatic hydrocarbon (PAH), serves as a structural backbone for various bioactive compounds. Its derivatives exhibit significant medicinal properties and have found applications in pharmaceuticals, dermatology, and research.
1. Anthracene In Medicine
Antimicrobial Properties: Some anthracene derivatives, such as anthraquinones, display antimicrobial activity against bacterial and fungal pathogens. These compounds are being explored for their potential use in antibiotic and antifungal drug development. Examples include anthraquinone derivatives like emodin and chrysophanol, which exhibit antibacterial and antifungal effects.
Anti-inflammatory Effects: Anthracene-based compounds have shown anti-inflammatory properties by modulating inflammatory pathways. They inhibit key inflammatory mediators, such as cytokines and prostaglandins, making them potential candidates for treating inflammatory diseases like arthritis and inflammatory bowel disease (IBD).
Anticancer Potential: Some anthracene derivatives, such as anthracyclines (e.g., doxorubicin, daunorubicin), are widely used as chemotherapeutic agents. These drugs intercalate into DNA, disrupting replication and transcription, which is particularly useful in cancer treatment.
2. Anthracene In Traditional Medicine
Herbal Remedies: Several medicinal plants contain naturally occurring anthraquinones, which have been used in traditional medicine for centuries. Extracts from these plants are often employed for their:
Laxative Effects: Natural anthraquinones, such as those found in Senna (Cassia species), Aloe vera, and Rhubarb (Rheum species), are well-known for their purgative and laxative effects. They stimulate peristalsis in the intestine and promote bowel movements.
Antioxidant and Detoxifying Properties: Traditional medicine systems, including Ayurveda and Traditional Chinese Medicine (TCM), use anthracene-containing plant extracts for detoxification and overall wellness.
3. Anthracene In Dermatology
Photodynamic Therapy (PDT): Certain anthracene derivatives, such as anthralin (dithranol), are widely used in dermatology for treating psoriasis and other skin disorders.
Mechanism of Action: Anthralin works by generating reactive oxygen species (ROS) and inhibiting excessive keratinocyte proliferation, thereby reducing plaque formation in psoriasis.
Other Dermatological Applications: Research is ongoing into anthracene-based compounds for their potential role in treating eczema, vitiligo, and other skin conditions.
4. Anthracene In Research and Development
Chemical Synthesis: Anthracene is a crucial precursor in the synthesis of various organic and pharmaceutical compounds. It plays a role in:
Drug Development: Many anthracene derivatives are synthesized for potential pharmacological applications, including antiviral, anticancer, and neuroprotective agents.
Molecular Probes: Anthracene-based compounds are used in molecular biology research, particularly in DNA-intercalating dyes for studying nucleic acids.
5. Anthracene As a Fluorescent Dye
Fluorescence Imaging: Anthracene and its derivatives exhibit strong fluorescence properties, making them valuable in:
Biomedical Imaging: Fluorescent anthracene derivatives are employed as probes for visualizing cellular structures and biochemical interactions in medical research.
Diagnostic Applications: These compounds are explored in diagnostic assays, including fluorescence-based detection methods for biomolecules and pathogens.
6. Anthracene in Environmental Monitoring
Biological Indicators: As a polycyclic aromatic hydrocarbon (PAH), anthracene is used in environmental and occupational health studies as a biomarker for PAH exposure.
Air and Water Quality Assessments: Monitoring anthracene levels in biological and environmental samples helps in assessing pollution and potential health risks associated with PAHs.
Toxicological Studies: Research into anthracene toxicity aids in understanding its impact on human health and the environment, leading to better safety regulations.
Safety Considerations of Anthracene
1. Toxicity: While certain derivatives may have therapeutic potential, the toxicity of anthracene-containing compounds needs consideration. Toxicological studies are essential to assess their safety profile.
2. Phototoxicity: Some anthracene derivatives, especially those used in dermatology and photodynamic therapy, may exhibit phototoxic effects. Adequate precautions are taken to minimize adverse reactions during light exposure.
3. Environmental Impact: Anthracene is a type of polycyclic aromatic hydrocarbon (PAH) found in the environment. PAHs can have environmental implications due to their persistence and potential toxicity.
4. Regulatory Measures: Regulatory measures may be in place to control the use of anthracene-containing compounds, especially in therapeutic applications, to ensure safety and minimize potential risks.
Anthracene and its derivatives showcase a range of pharmacological properties, from antimicrobial and anti-inflammatory effects to applications in dermatology and fluorescence imaging. However, safety considerations, including toxicity and environmental impact, highlight the importance of rigorous safety assessments and regulatory oversight. Balancing the potential benefits of anthracene in medicine with the need for safety measures is crucial for advancing its applications in various fields.
Visit also: Pharmacareerinsider.com

