Atomic Absorption Spectroscopy (AAS) is an essential and widely utilized analytical technique that plays a crucial role in determining the concentration of metal elements in various types of samples. It is particularly renowned for its high precision, accuracy, and sensitivity in detecting trace amounts of metals in complex matrices. The technique finds extensive applications in a diverse range of fields, including environmental monitoring, pharmaceutical research, food and agriculture, clinical diagnostics, metallurgy, and forensic science. The ability of AAS to provide quantitative data on metallic elements makes it an invaluable tool in modern analytical laboratories, allowing for strict quality control, contamination detection, and compliance with regulatory standards.
Suggested Post: Flame Emission Spectroscopy
Principle of Atomic Absorption Spectroscopy
The working principle of AAS is based on the concept that free atoms in their ground state have a unique ability to absorb radiation at characteristic wavelengths. This absorption occurs when atoms of a specific metal are subjected to electromagnetic radiation of a precise wavelength that corresponds to the energy required for electronic excitation. By measuring the amount of absorbed radiation, the concentration of the metal in the sample can be accurately determined.
The key steps involved in Atomic Absorption Spectroscopy include:
- Atomization: The sample containing metal ions is introduced into a high-temperature source, such as a flame or graphite furnace. The intense heat energy breaks down the chemical bonds in the sample, converting the metal ions into their neutral atomic state.
- Absorption of Radiation: A specific light source, typically a Hollow Cathode Lamp (HCL) or an Electrodeless Discharge Lamp (EDL), emits monochromatic light at the characteristic wavelength of the target element. The free atoms present in the sample absorb this light selectively.
- Measurement of Absorbance: The reduction in the intensity of the transmitted light, due to absorption by the atoms, is measured. The amount of absorbed radiation is directly proportional to the concentration of the target element, following Beer-Lambert’s Law:
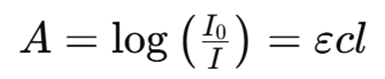
where:
A = is the absorbance,
Io = is the intensity of incident light,
I = is the intensity of transmitted light,
ε = is the molar absorptivity,
c = is the concentration of the analyte,
l = is the path length of the light.
The absorbance values obtained for the sample are compared with those from standard solutions of known concentrations to derive the precise concentration of the metal in the sample.
Instrumentation of Atomic Absorption Spectroscopy
A typical Atomic Absorption Spectroscopy setup comprises multiple essential components that work in unison to ensure accurate and reliable analysis. The major components of an AAS instrument are as follows:
1. Radiation Source
The light source used in AAS must emit radiation at a wavelength characteristic of the element being analyzed. The two most commonly used sources are:
- Hollow Cathode Lamp (HCL): This is the most frequently used light source in AAS. It consists of a cathode made from the element to be analyzed and an anode within a glass tube filled with inert gas (usually argon or neon). When an electrical discharge is applied, the cathode material emits the characteristic radiation of the target element.
- Electrodeless Discharge Lamp (EDL): Used when higher-intensity radiation is needed, especially for elements with weak emission lines. This lamp contains a sealed quartz tube with a small quantity of the target element and is excited by radio frequency or microwave energy.
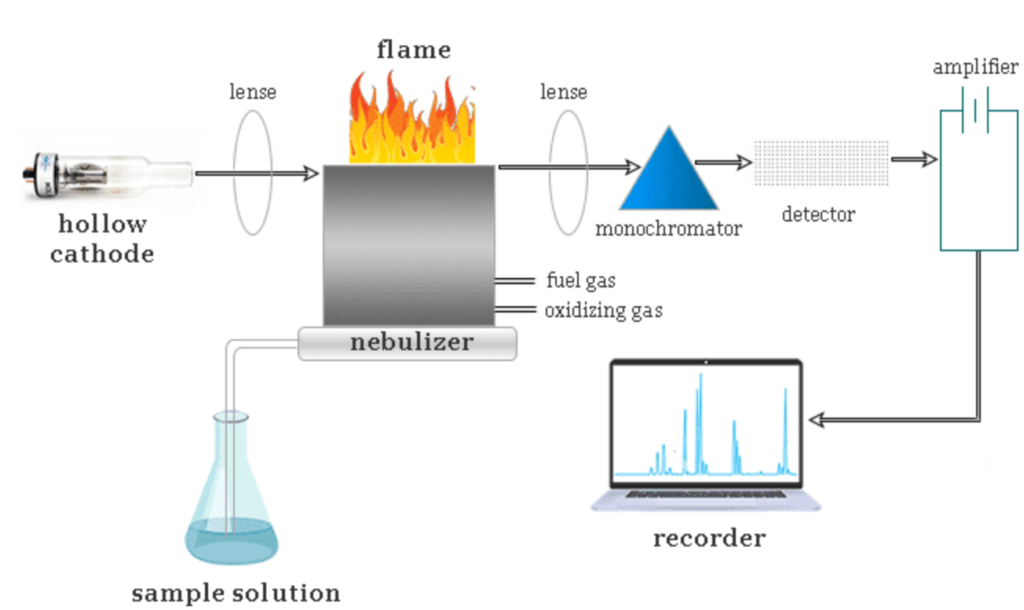
2. Atomization System
To measure absorption, the sample must be converted into free atoms, a process known as atomization. The two main atomization techniques in AAS are:
- Flame Atomizer: Involves a burner where the sample is nebulized into a fine mist and mixed with a fuel-oxidant flame (such as acetylene-air or acetylene-nitrous oxide). The high temperature of the flame converts metal ions into free atoms, allowing for absorption measurement.
- Graphite Furnace Atomizer: Offers enhanced sensitivity by heating the sample in a graphite tube in a stepwise manner (drying, ashing, and atomization). This method is highly effective for analyzing trace elements at very low concentrations.
- Hydride Generation System: Used for elements such as arsenic (As) and selenium (Se), where volatile hydrides of the metal are formed and transported to a detection system, leading to improved sensitivity.
3. Monochromator
The monochromator is an optical device that selectively isolates the desired wavelength of light from the radiation source while filtering out any other interfering wavelengths. Common monochromators include diffraction gratings and prism-based systems, which ensure that only the specific spectral line of interest reaches the detector.
4. Detector
The role of the detector is to measure the intensity of transmitted light and convert it into an electrical signal. The most commonly used detector in AAS is the Photomultiplier Tube (PMT), which is highly sensitive and capable of detecting very low-intensity signals.
5. Data Processing System
The electrical signals generated by the detector are processed and analyzed by a microprocessor or computer system. The absorbance values are recorded, and the concentration of the target metal is calculated based on calibration curves derived from standard solutions.
Interferences in Atomic Absorption Spectroscopy
Despite its high accuracy, AAS is subject to various interferences that can impact the measurement results. The main types of interferences include:
1. Spectral Interference: Occurs when other substances present in the sample absorb light at the same wavelength as the analyte, leading to erroneous results.Mitigated by using background correction techniques such as Deuterium Lamp Background Correction and Zeeman Effect Background Correction.
2. Chemical Interference: Occurs when chemical interactions prevent the complete atomization of the analyte.Controlled by adding chemical agents known as releasing agents or matrix modifiers to break interfering complexes and enhance free atom formation.
3. Ionization Interference: Caused by high temperatures leading to the ionization of free atoms, reducing their ability to absorb radiation.Minimized by introducing ionization suppressants, such as potassium or cesium, which donate free electrons to stabilize the atomic state of the analyte.
4. Matrix Effects: Arises due to variations in sample composition affecting atomization efficiency.Minimized by using matrix-matched calibration standards or employing the standard addition method.
5. Physical Interference: Occurs due to variations in viscosity, surface tension, or nebulization efficiency of the sample.Controlled by maintaining uniform sample preparation and dilution.
Applications of Atomic Absorption Spectroscopy
AAS has diverse applications across multiple scientific and industrial domains. Some key applications include:
- Environmental Analysis: Detecting toxic metals like lead (Pb), arsenic (As), and mercury (Hg) in water, soil, and air.
- Clinical Diagnostics: Measuring trace elements in biological fluids to monitor nutritional deficiencies or toxic metal exposure.
- Pharmaceutical Analysis: Ensuring the quality of drugs by detecting metal impurities.
- Food and Agriculture: Assessing the presence of essential and toxic metals in food products and fertilizers.
- Metallurgy and Industry: Quality control of alloys and raw materials used in manufacturing.
Conclusion
Atomic Absorption Spectroscopy remains one of the most reliable and extensively used techniques for the quantitative determination of metal elements. Its ability to provide accurate, sensitive, and precise results makes it an indispensable tool for scientific research, industrial applications, and regulatory compliance. Continuous advancements in AAS technology continue to enhance its efficiency and applicability across a wide range of disciplines.

