Bromometry is an analytical method that involves the quantitative determination of substances in a sample by using bromine as the titrant. In this note, we will explore the principles, procedures, and applications of bromometry.
1. Principles of Bromometry
Bromometry is based on the oxidation-reduction reaction involving bromine. The oxidizing power of bromine is utilized to react with analytes such as phenols, organic compounds, and certain metal ions.
The reaction typically follows this mechanism:
Generation of Bromine in Acidic Medium:

In this reaction, potassium bromate (KBrO₃) acts as a source of bromine in the presence of potassium bromide (KBr) and an acid.
Reaction with the Analyte:
The liberated bromine reacts with the substance to be analyzed.
Example: If an organic compound like phenol is analyzed, bromine reacts with the phenol to form brominated derivatives.
Detection of End Point:
The excess bromine can be detected using starch-iodide paper, methyl orange, or by adding an excess of potassium iodide (KI) to react with the remaining bromine, forming iodine.
The liberated iodine is then titrated with sodium thiosulfate (Na2S2O3).
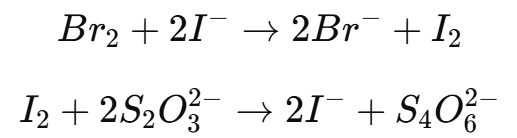
2. Applications of Bromometry
Quantification of Analytes
Chemists widely use bromometry to determine the concentration of various analytes, such as thiosulfates, phenols, and unsaturated compounds, in various samples, including water, pharmaceuticals, and environmental matrices.
Quality Control
It plays a crucial role in quality control processes across industries where accurate measurements of analytes are vital for product quality and safety.
3. Procedure for Bromometry
Bromometry is a redox titration method used to determine compounds that react with bromine (Br₂). The procedure typically involves generating bromine in situ using potassium bromate (KBrO₃) in an acidic medium, followed by its reaction with the analyte.
General Bromometric Titration Procedure:
Step 1: Preparation of Bromine In Situ
- In a conical flask, take a known volume of the sample containing the analyte.
- Add potassium bromide (KBr) solution to provide bromide ions.
- Add dilute sulfuric acid (H₂SO₄) or hydrochloric acid (HCl) to create an acidic medium.
- Introduce a known volume of potassium bromate (KBrO₃) solution, which will generate bromine in situ:
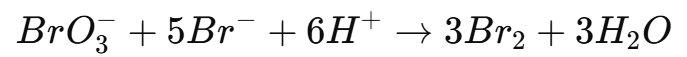
5. The bromine liberated will oxidize the analyte.
Step 2: Titration with Sodium Thiosulfate (if necessary)
6. If excess bromine remains, add potassium iodide (KI) solution to react with the unreacted bromine:
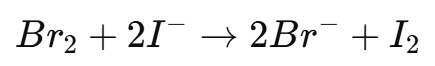
7. The liberated iodine (I2I_2I2) is then titrated against sodium thiosulfate (Na2S2O3Na_2S_2O_3Na2S2O3) until the brown color of iodine fades to pale yellow.
8. Add a few drops of starch indicator, which will turn the solution deep blue due to the formation of the starch-iodine complex.
9. Continue titrating with sodium thiosulfate until the blue color disappears, indicating the endpoint.
End-Point Detection:
- The disappearance of the blue color (from starch-iodine complex) indicates the endpoint.
- If a direct reaction occurs without iodine liberation, fading of the bromine color can serve as the endpoint.
Calculation:
The amount of analyte present is determined using the reaction stoichiometry:

From the balanced reaction equation, the required factor for the analyte is used to determine its concentration.
4. Significance of Bromometry
High Sensitivity: Bromometry is known for its high sensitivity, allowing the quantification of analytes, even at low concentrations.
Versatility: You can apply it to various analytes, making it a valuable tool in different industries, including water analysis, pharmaceuticals, and chemical manufacturing.
Quantitative Analysis: Bromometry provides quantitative data, making it an essential technique in research, quality control, and regulatory compliance.
5. Examples of Bromometry
Measurement of Thiosulfate in Photographic Processing: Use bromometry to determine the concentration of thiosulfate in photographic solutions, ensuring the proper fixing of images.
Analysis of Phenols in Water: Employ it to quantify phenolic compounds in water samples and to assess water quality and environmental compliance.
Visit to: Pharmacareerinsider.com

