Cycloalkanes constitute a category of organic compounds distinguished by the presence of one or more carbon-carbon single bonds organized in a closed ring or cyclic structure. This group falls within the broader category of alkanes, which are saturated hydrocarbons composed of carbon and hydrogen atoms linked together exclusively by single bonds. The general formula for cycloalkanes is (CnH2n), where ‘n’ denotes the number of carbon atoms forming the ring. The inaugural member of cycloalkanes is Cyclopropane, with the formula C3H6. These compounds are frequently encountered in nature and intentionally synthesized in laboratories to serve diverse applications.
Nomenclature of Cycloalkanes
1. Simple Cycloalkanes:
– The addition of the prefix “cyclo” to the alkane name corresponds to the number of carbon atoms present in the ring for single-ring cycloalkanes.
– Subsequently, the name is supplemented with the suffix “-ane” to signify that the compound falls under the category of alkanes.
– The selection of the prefix is determined by the number of carbon atoms forming the ring:
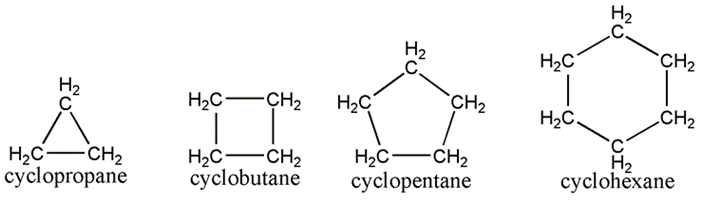
– Cyclopropane: Comprising 3 carbon atoms in the ring
– Cyclobutane: Comprising 4 carbon atoms in the ring
– Cyclopentane: Comprising 5 carbon atoms in the ring
– Cyclohexane: Comprising 6 carbon atoms in the ring
– Cycloheptane: Comprising 7 carbon atoms in the ring
– Cyclooctane: Comprising 8 carbon atoms in the ring
– For cycloalkanes with more than eight carbons, a similar nomenclature is employed, using the appropriate prefix corresponding to the number of carbon atoms in the ring.
2. Substituted Cycloalkanes:
– If substituents are present on the cycloalkane ring, they are named using the common system.
– The substituent names are listed alphabetically before the parent cycloalkane name.
– The position of each substituent is indicated by assigning a number to each carbon atom in the ring, starting with the carbon atom that gives the lowest set of locants to the substituents.
– If multiple substituents are present, the prefixes “di-,” “tri-,” etc., indicate the number of identical substituents.
– If different substituents are present, they are listed in alphabetical order.
3. Examples:
– 1-Methylcyclohexane: Cyclohexane with a methyl substituent on carbon atom 1.
– 1,3-Dimethylcyclopentane: Cyclopentane with methyl substituents on carbon atoms 1 and 3.
– 1-Ethyl-3-methylcyclohexane: Cyclohexane with ethyl substituent on carbon atom 1 and methyl substituent on carbon atom 3.

