Dichrometry and Titration with Potassium
Dichrometry and Titration with Potassium: Dichrometry is an analytical method that quantitatively determines reducing agents in a sample using a dichromate solution as the titrant. Conduct the titration until you reach a specific endpoint, often signaled by a color change. In this note, we will explore the principles, procedures, and applications of dichrometry, particularly in titration with potassium iodate.
1. Principles of Dichrometry and Titration with Potassium
Dichromate as an Oxidizing Agent
In dichrometry, you employ a solution of potassium dichromate (K₂Cr₂O₃) as an oxidizing agent. The analyte (reducing agent) in the sample reduces this dichromate.
The basis of dichrometry lies in the redox reaction between the reducing agent in the sample and the dichromate. You determine the endpoint through the complete reaction of the reducing agent with the dichromate, typically indicated by a color change.
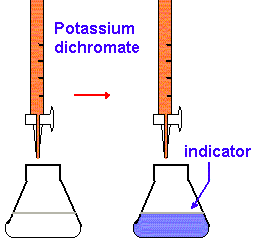
2. Applications of Dichrometry with Potassium Iodate
Quantification of Reducing Agents
Chemists commonly use dichrometry to determine the concentration of various reducing agents, such as hydrogen peroxide, iron, and organic compounds, in various samples, including environmental samples, pharmaceuticals, and food products.
Quality Control
It plays a crucial role in quality control processes, ensuring product quality, compliance with regulatory standards, and safety in various industries.
3. Procedure for Dichrometry with Potassium Iodate
Sample Preparation: The sample is carefully prepared to ensure that the reducing agent of interest is suitable for titration.
Formation of Dichromate Solution: A potassium dichromate solution is prepared and standardized. This solution serves as the titrant in the titration.
Titration: Titrates the dichromate solution into the sample containing the reducing agent. You reach the endpoint when all the reducing agent has reacted with the dichromate, resulting in a color change that signals the endpoint.
Calculations: Use the volume and concentration of the dichromate solution used to reach the endpoint to calculate the concentration of the reducing agent in the sample.
4. Significance of Dichrometry and Titration with Potassium
Sensitivity: Dichrometry is known for its sensitivity, which allows it to quantify reducing agents, even at low concentrations.
Versatility: You can apply it to a wide range of reducing agents, making it a valuable tool in different industries, including environmental analysis, pharmaceuticals, and chemical manufacturing.
Quantitative Analysis: Dichrometry provides quantitative data, making it an essential technique in research, quality control, and regulatory compliance.
5. Example of Dichrometry and Titration with Potassium
1. Dichrometry (Redox Titration Using Dichromate)
Dichrometry refers to oxidation-reduction (redox) titrations involving potassium dichromate (K₂Cr₂O₇) as an oxidizing agent. It is commonly used for determining the concentration of reducing agents such as ferrous iron (Fe²⁺), oxalates, or sulfites.
Example: Determination of Ferrous Iron (Fe²⁺) Using Dichromate

Indicator: Diphenylamine sulfonate or barium diphenylamine sulfonate (end-point color change: violet to green).
Procedure:
- Prepare a standard solution of K₂Cr₂O₇.
- Take the ferrous iron (Fe²⁺) solution in an acidic medium (using H₂SO₄).
- Add the dichromate solution from the burette until a permanent color change is observed.
- Calculate Fe²⁺ concentration using the stoichiometry of the reaction.
2. Potassium Iodate (KIO₃) Titration
Potassium iodate (KIO₃) is a primary standard oxidizing agent used in iodometric titrations.
Example: Standardization of Sodium Thiosulfate (Na₂S₂O₃) Using KIO₃
Reaction Steps:
- KIO₃ reacts with excess KI in acidic medium to liberate iodine (I₂):

2. The liberated iodine is titrated with sodium thiosulfate (Na₂S₂O₃):

Indicator: Starch solution (end-point color change: blue to colorless).
Procedure:
- Dissolve a known amount of KIO₃ in water.
- Add excess KI and HCl to generate I₂.
- Titrate with Na₂S₂O₃ until the solution turns pale yellow.
- Add starch indicator and continue titration until the blue color disappears.
- Calculate the molarity of Na₂S₂O₃ based on KIO₃ reaction.
These titrations are widely used in pharmaceutical, environmental, and analytical chemistry for quantitative analysis.

