Difference and derivative spectroscopy are advanced techniques in UV-Visible spectrophotometry that enhance spectral analysis by improving resolution, eliminating background interferences, and providing more precise quantitative measurements. These methods are particularly useful for the analysis of complex mixtures, detecting minor spectral variations, and studying molecular interactions. The increasing complexity of pharmaceutical formulations, environmental samples, and biological matrices has necessitated the use of sophisticated spectroscopic techniques, making difference and derivative spectroscopy indispensable tools in analytical chemistry.
Difference Spectroscopy
Definition and Principle
Difference spectroscopy is a technique that measures the difference between two absorption spectra obtained under different experimental conditions, such as pH variation, temperature change, or chemical modification. This method effectively eliminates baseline noise and enhances the detection of small spectral changes, making it highly beneficial in pharmaceutical and biochemical analyses.
The principle of difference spectroscopy is based on the subtraction of one spectrum from another, revealing only the changes induced by a specific factor. This approach enhances the visibility of spectral shifts, peak broadening, and fine structural changes that may not be apparent in conventional absorption spectra. By analyzing these spectral differences, researchers can gain insights into molecular interactions, conformational changes, and reaction mechanisms.
Experimental Considerations
To perform difference spectroscopy, it is essential to ensure that:
- The reference and sample solutions are prepared under identical conditions except for the variable being studied.
- High-precision spectrophotometers are used to minimize instrumental noise.
- Proper baseline correction is applied to eliminate unwanted variations.
- The difference spectrum is interpreted carefully, considering solvent effects, path length consistency, and potential spectral overlaps.
Applications of Difference Spectroscopy
- Pharmaceutical Analysis – Used for determining drug stability, degradation pathways, and formulation consistency. Many active pharmaceutical ingredients (APIs) exhibit subtle spectral changes upon degradation or polymorphic transitions, which can be detected using difference spectroscopy.
- Protein and Enzyme Studies – Helps in identifying conformational changes, ligand binding, and denaturation processes. Difference spectra can reveal alterations in protein secondary and tertiary structures caused by external stimuli like heat, pH, or ligand binding.
- pH-Dependent Studies – Assists in analyzing ionization states of molecules by comparing spectra at different pH values. This is particularly useful for understanding the pKa values of drugs and optimizing formulation conditions.
- Environmental Monitoring – Useful in detecting pollutants and their interactions in aqueous solutions. Many pollutants undergo chemical transformations under different environmental conditions, and difference spectroscopy can help in studying these changes.
- Study of Redox Reactions – Redox reactions involve changes in oxidation states, which often result in subtle shifts in absorption spectra. Difference spectroscopy can be employed to investigate these redox transformations, particularly in coordination chemistry and biochemical redox reactions.
Advantages of Difference Spectroscopy
- Enhances sensitivity by removing background absorption and unrelated spectral components.
- Allows detection of minor spectral shifts and molecular interactions.
- Improves selectivity in multi-component analysis by distinguishing overlapping spectra.
- Provides insight into chemical and structural modifications of molecules.
Limitations of Difference Spectroscopy
- Requires precise control over experimental conditions to ensure reproducibility.
- Not suitable for highly unstable compounds that degrade rapidly.
- May require complex data processing techniques and computational corrections to remove instrumental artifacts.
Derivative Spectroscopy
Definition and Principle
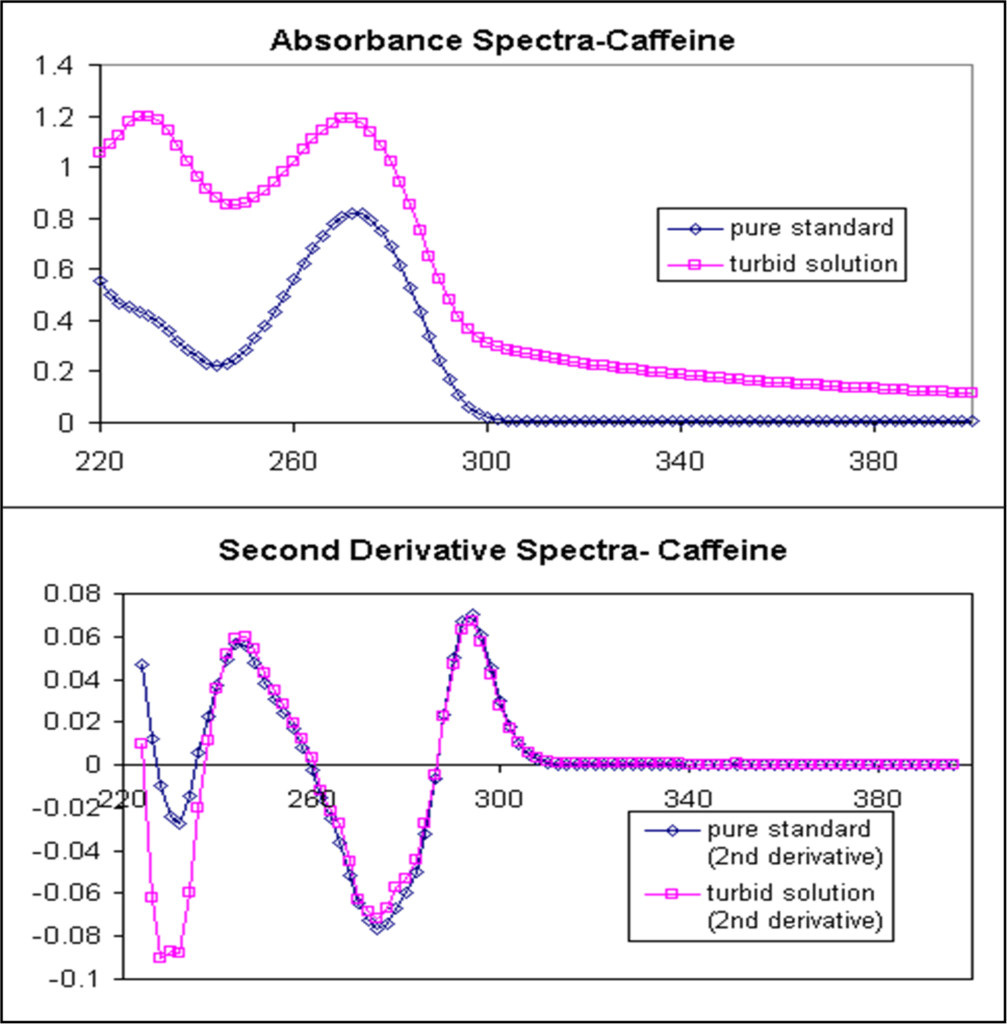
Derivative spectroscopy involves the mathematical transformation of an absorption spectrum to obtain its first, second, third, or higher-order derivative spectra. This transformation enhances spectral resolution by sharpening peaks, reducing background interference, and separating overlapping bands. The ability to resolve closely spaced peaks makes derivative spectroscopy highly useful for complex sample analysis.
The first derivative spectrum (D¹) represents the rate of change of absorbance with respect to wavelength, showing peaks and troughs corresponding to inflection points in the original spectrum. The second derivative spectrum (D²) emphasizes peak curvature, highlighting minor spectral features and reducing baseline shifts. Higher-order derivatives (D³, D⁴) further enhance spectral resolution but may introduce noise due to increased sensitivity to small fluctuations.
Mathematical Expression
The derivative of an absorbance spectrum is expressed as:
- First derivative: – Identifies changes in slope and highlights minor peak variations.
- Second derivative: – Enhances the resolution of overlapping peaks and reduces baseline drift.
- Higher derivatives: , where is the derivative order – Used for specialized applications requiring extreme peak sharpening and noise filtration.
Experimental Considerations
- Spectral smoothing techniques, such as Savitzky-Golay filtering, are often applied to reduce noise.
- The choice of derivative order depends on the complexity of the sample and the degree of resolution required.
- Instrumental precision and data acquisition speed play a crucial role in obtaining accurate derivative spectra.
Applications of Derivative Spectroscopy
- Quantitative Analysis – Used for the determination of overlapping peaks in complex mixtures, allowing for accurate quantification of components in multi-component formulations.
- Pharmaceutical Quality Control – Aids in identifying impurities, degradation products, and polymorphic forms of drugs.
- Biomedical Research – Helps in studying biomolecules such as proteins, DNA, and enzymes, especially in detecting conformational changes and molecular interactions.
- Food and Beverage Industry – Applied in detecting adulterants, colorants, and quality parameters of food products.
- Forensic Analysis – Useful in detecting trace amounts of drugs, toxins, and illegal substances, enhancing forensic investigations.
- Nanomaterial Characterization – Derivative spectroscopy is increasingly used in the study of nanomaterials, where minor shifts in spectral features provide insights into particle size, aggregation, and surface modifications.
Advantages of Derivative Spectroscopy
- Enhances spectral resolution by minimizing overlapping bands and improving peak separation.
- Removes baseline shifts, improving accuracy in quantitative analysis.
- Provides better peak separation, aiding in the analysis of complex mixtures.
- Useful for detecting hidden spectral features and subtle molecular interactions.
Limitations of Derivative Spectroscopy
- Higher-order derivatives may introduce noise, reducing signal clarity and requiring additional smoothing techniques.
- Requires sophisticated data processing techniques and high computational power.
- Sensitive to instrumental variations and sample preparation inconsistencies, demanding precise experimental conditions.
Comparison Between Difference and Derivative Spectroscopy
| Feature | Difference Spectroscopy | Derivative Spectroscopy |
| Principle | Subtraction of two spectra | Derivation of spectral curve |
| Application Focus | Detecting minor spectral changes | Enhancing peak resolution and separation |
| Sensitivity | High for small changes | High for overlapping peaks |
| Data Processing | Requires careful baseline correction | Involves mathematical transformations |
| Use in Pharmaceuticals | Stability studies, drug formulation analysis | Impurity detection, multi-component analysis |
| Instrumentation Requirements | Standard UV-Vis spectrophotometer | Requires software for derivative calculations |
Instrumentation and Software for Derivative Spectroscopy
Derivative spectroscopy requires spectrophotometers equipped with advanced data processing software. Many modern UV-Vis spectrophotometers offer built-in derivative analysis functions, enabling real-time spectral transformation and analysis. Software such as MATLAB, Origin, and specialized spectroscopic tools allow researchers to apply derivative functions, smooth spectra, and enhance data interpretation.
Conclusion
Difference and derivative spectroscopy are powerful analytical techniques in UV-Visible spectrophotometry, offering enhanced resolution, improved sensitivity, and better spectral interpretation. Difference spectroscopy is particularly useful for detecting subtle spectral changes, while derivative spectroscopy is essential for peak separation and quantification in complex samples. Despite their limitations, these methods remain indispensable in pharmaceutical, biomedical, environmental, and forensic research, contributing significantly to advancements in analytical sciences.

