Disaccharides, a type of carbohydrate, consist of two monosaccharide units linked together by a glycosidic bond. Monosaccharides, the building blocks for disaccharides, undergo a condensation reaction to form a disaccharide, eliminating a water molecule as the two monosaccharide units join together.
Maltose
Maltose is a disaccharide, a type of carbohydrate consisting of two glucose molecules linked together by an α-1,4-glycosidic bond. Commonly known as malt sugar, maltose is produced during the hydrolysis of starch, particularly in germinating seeds, and forms a component of malted grains such as barley.
In its crystalline form, maltose is a white, water-soluble powder with a sweet taste. The digestive enzyme maltase breaks down maltose into its constituent glucose molecules during the digestive process. Various food and beverage products, particularly in the brewing industry, use maltose. It is a product of the breakdown of starch in malted grains and contributes to the fermentation process in beer production.
The structural formula for maltose can be represented as follows:

In this representation:
“C” represents a carbon atom.
“H” represents a hydrogen atom.
“O” represents an oxygen atom.
“||” represents the α-1,4-glycosidic bond.
Each vertical line between carbon atoms represents a single covalent bond.
This structure illustrates the linkage between the carbon atom at the first position of one glucose molecule and the carbon atom at the fourth position of the other glucose molecule, forming the glycosidic bond. During the enzymatic breakdown of starch, germinating seeds commonly contain maltose. It is a source of glucose upon hydrolysis in the digestive system.
Lactose
The Lactose is a disaccharide, a type of carbohydrate, composed of one molecule of glucose and one molecule of galactose linked together by a β-1,4-glycosidic bond. It is commonly found in the milk of mammals and is often referred to as milk sugar. Lactose is a significant energy source for infants, and the enzyme lactase facilitates digestion.
In its crystalline form, lactose is a white, water-soluble powder with a mildly sweet taste. The digestive enzyme lactase breaks down lactose into its constituent monosaccharides, glucose, and galactose, during the digestive process. Some individuals, particularly those with lactose intolerance, have reduced levels of lactase, leading to difficulty digesting lactose and resulting in symptoms such as bloating and gastrointestinal discomfort after consuming dairy products.
Various food products commonly use lactose as an ingredient, and there are available lactose-free alternatives for individuals who have difficulty digesting this disaccharide.

In this representation:
“C” represents a carbon atom.
“H” represents a hydrogen atom.
“O” represents an oxygen atom.
“||” represents the β-1,4-glycosidic bond.
Each vertical line between carbon atoms represents a single covalent bond.
This structure illustrates the linkage between the carbon atom at the first position of glucose and the carbon atom at the fourth position of galactose, forming the glycosidic bond.Lactose, found commonly in milk, serves as a source of energy for infants. The enzyme lactase is necessary to break down lactose into its constituent glucose and galactose molecules during digestion.
Sucrose
The Sucrose is a disaccharide, a type of carbohydrate, that consists of one molecule of glucose and one molecule of fructose linked together by an α,β-1,2-glycosidic bond. Many plants, especially sugar cane and sugar beets, contain sucrose, a common form of sugar. It is the most widely used sugar for human consumption and is commonly known as table sugar.
In its crystalline form, sucrose manifests as a white, odorless, and sweet-tasting powder or solid. It serves as a major source of dietary energy and finds common use as a sweetener in various foods and beverages. In the digestive system, sucrose undergoes hydrolysis facilitated by the enzyme sucrase, breaking down into its constituent monosaccharides, glucose, and fructose. These monosaccharides can then be absorbed and utilized by the body for energy.
The structural formula for sucrose can be represented as follows:
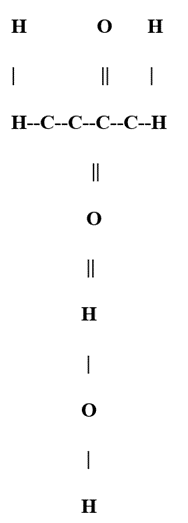
In this representation:
“C” represents a carbon atom.
“H” represents a hydrogen atom.
“O” represents an oxygen atom.
“||” represents the α,β-1,2-glycosidic bond.
Each vertical line between carbon atoms represents a single covalent bond.
This structure illustrates the linkage between the carbon atom at the first position of glucose and the carbon atom at the second position of fructose, forming the glycosidic bond. Commonly known as table sugar, sucrose is found in various plants, particularly in sugar cane and sugar beets. During digestion, the enzyme sucrase breaks sucrose down into its constituent glucose and fructose molecules.

