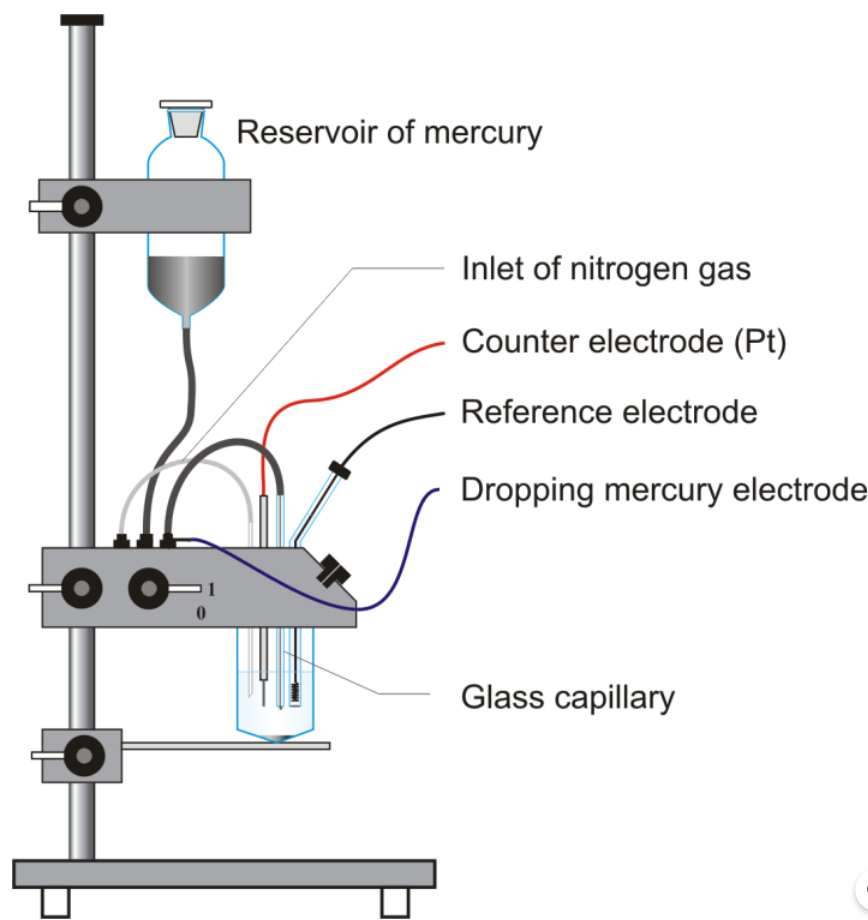
Analytical chemists use a Dropping Mercury Electrode (DME) as an electrochemical instrument, especially for investigating various electrochemical reactions. It consists of a small droplet of mercury continuously replenished by a controlled flow of mercury from a reservoir. Here’s how it is constructed and works:
Construction
1. Mercury Reservoir: The DME has a reservoir holding a larger mercury volume. Typically, researchers make this reservoir from glass or other chemically inert materials.
2. Mercury Delivery System: A controlled delivery system allows a precise amount of mercury to flow from the reservoir to the electrode. A fine capillary tube or other mechanisms can deliver the mercury.
3. Electrode Body: The DME has an electrode body usually made of chemically inert materials like glass or quartz. The electrode body holds the mercury droplet and provides electrical contact to the mercury.
4. Reference Electrode: Researchers often use a reference electrode in conjunction with the DME to accurately measure potential. Common reference electrodes include the saturated calomel electrode (SCE) or the silver/silver chloride electrode.
Working
1. Mercury Droplet Formation: Initially, a small droplet of mercury is formed at the tip of the electrode. This droplet acts as the working electrode.
2. Droplet Control: Carefully controlled, the mercury flow from the reservoir ensures a steady replenishment of the mercury droplet. This controlled flow is essential for the DME’s operation.
3. Electrochemical Reactions: Electrochemical reactions occur at the mercury droplet’s surface. Various analytes and substances can be studied by introducing them into the electrolyte immersed in the DME. The potential and current at the electrode are measured during these reactions.
4. Steady-State Operation: The DME operates under steady-state conditions because it continuously replenishes the mercury droplet. This allows for precise and stable measurements over time.
5. Applications: Researchers use DMEs in various electrochemical experiments, such as cyclic voltammetry, polarography, and other electroanalytical techniques.They are particularly useful for studying redox reactions and the determination of analytes in solution.
The advantages of DMEs include their ability to provide a reproducible and stable working electrode surface and their low background current. However, the handling of mercury, which is toxic, poses environmental and safety concerns. As a result, there is a growing interest in developing alternative working electrodes that do not use mercury, such as carbon-based electrodes or solid-state materials, for similar electrochemical applications.




