Introduction:
Electrophoresis is a powerful analytical technique used to separate charged molecules, such as proteins, DNA, and RNA, based on their size, shape, and charge under the influence of an electric field. When an electric current is applied to a solution containing charged particles, these molecules move toward the electrode with the opposite charge. It was first developed by Arne Tiselius in 1937 for separating proteins.
The speed and direction of this movement are determined by the charge on the molecule and the medium through which it travels. Smaller, highly charged molecules move faster, while larger or less charged molecules move slower. The separated molecules form distinct bands, which can be visualized and analyzed.
Electrophoresis is used in a variety of fields, including molecular biology, biochemistry, and forensic science, for tasks such as:
- DNA fingerprinting
- Protein separation
- Genetic research
- Diagnosing diseases
PRINCIPLE OF ELECTROPHORESIS:
Electrophoresis is based on the movement of charged molecules in an electric field. When an electric field is applied, charged particles migrate toward the electrode with an opposite charge—cations (positively charged molecules) move toward the cathode, while anions (negatively charged molecules) move toward the anode. The rate of migration depends on several factors:
- Applied Voltage (E): Higher voltage increases the rate of movement.
- Charge on the Molecule (q): Molecules with a higher net charge move faster.
- Molecular Size and Shape: Larger molecules experience more friction and thusmove slower through the medium.
- Medium Viscosity: The more viscous the medium, the slower the movement.
- Temperature: Higher temperatures can reduce the medium’s viscosity but may also affect molecule stability.
The general equation governing this is:
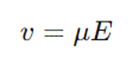
Where,
v = velocity of the particle
μ = electrophoretic mobility (determined by charge and resistance)
E = applied electric field strength.
Electrophoretic mobility (μ) is positive for cations and negative for anions.
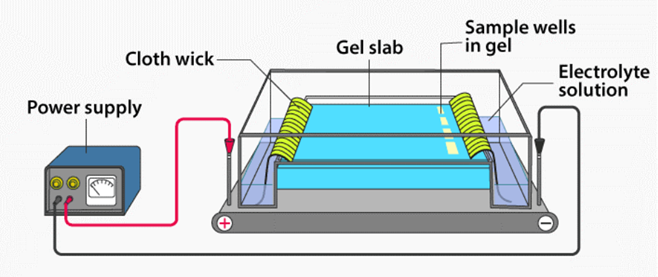
METHODOLOGY OF ELECTROPHORESIS:
- Sample Preparation: The sample (proteins, DNA, or RNA) is mixed with a buffer solution to maintain a stable pH and is loaded onto a medium such as gel or paper.
- Application of Electric Field: An electric field is applied across the medium, causing the charged molecules to move toward their respective electrodes. Positively charged molecules migrate toward the cathode (negative electrode), while negatively charged molecules move toward the anode (positive electrode).
- Separation of Molecules: Molecules separate based on their size, charge, and shape. Smaller or more charged molecules migrate faster, while larger molecules encounter more resistance and move slower.
- Visualization of Results: After electrophoresis, the separated molecules are visualized using specific stains (e.g., ethidium bromide for DNA) or under UV light. This allows the formation of distinct bands, each representing a different molecule in the sample.
- Analysis: The position and intensity of the bands provide information about the molecular size, charge, and quantity of the molecules in the sample.
FACTORS AFFECTING ELECTROPHORETIC MOBILITY:
1. Charge of the Molecule: Molecules with a higher charge move faster in an electric field. An increase in the charge increases the force exerted on the molecule, speeding up its migration.
2. Size and Shape of the Molecule: Larger molecules encounter more resistance from the medium and move slower than smaller molecules. The shape also affects how easily a molecule can move through the medium.
3. Medium (Matrix) Properties: The type and concentration of the medium used (gel, paper, etc.) play a crucial role in molecular separation. For instance, denser gels like agarose or polyacrylamide provide more resistance, slowing down larger molecules more than smaller ones.
4. pH of the Buffer: The pH of the buffer affects the ionization state of the molecules. At different pH levels, molecules may acquire more or less charge, influencing their mobility.
5. Strength of the Electric Field: The voltage applied during electrophoresis influences the movement of molecules. Higher voltages lead to faster movement, but excessively high voltages can cause heat generation, which may denature proteins or affect the separation process.
6. Temperature: As the temperature increases, molecular mobility may increase. However, excessive heat can cause the medium to degrade or distort results by denaturing the molecules being separated.
7. Ionic Strength of the Buffer:
– A buffer with a high ionic strength minimizes heat generation and ensures stable separation by conducting the current better.
TECHNIQUES OF ELECTROPHORESIS
1. Paper Electrophoresis:
In paper electrophoresis, a strip of cellulose paper is used as the supporting medium. The sample is applied onto the paper, and an electric field is applied across it.
Mechanism: Charged molecules migrate along the paper based on their charge-to-mass ratio.
Advantages: It is simple and cost-effective, widely used for separating small biomolecules like amino acids, peptides, and small ions.
Disadvantages: Poor resolution for large or complex molecules; not suitable for high-resolution separations.
2. Gel Electrophoresis:
(a) Agarose Gel Electrophoresis:
Agarose gel is commonly used for DNA and RNA separation. The gel has large pores, allowing separation based primarily on the size of the molecule.
Mechanism: Smaller molecules move faster through the gel matrix, while larger molecules are retarded.
Advantages: High resolution, suitable for DNA, RNA, and large biomolecules; visualized using dyes and UV light.
Applications: Genetic analysis, PCR product separation, and sequencing.
(b) Polyacrylamide Gel Electrophoresis (PAGE):
Polyacrylamide gel has smaller pores than agarose, making it suitable for separating proteins and smaller nucleic acids.
SDS-PAGE (Sodium Dodecyl Sulfate-PAGE): SDS is added to denature proteins and give them a uniform negative charge, allowing separation based purely on size.
Native PAGE: Proteins are separated without denaturation, preserving their native structure and activity.
Advantages: Provides high resolution for proteins and peptides.
Applications: Protein purification, molecular weight determination, and enzyme activity studies.
3. Capillary Electrophoresis (CE):
A high-resolution technique where separation occurs in a thin capillary tube filled with an electrolyte.
Mechanism: When a voltage is applied across the capillary, molecules migrate through based on their charge-to-size ratio. It offers superior resolution because of the thin capillary and the small sample volume used.
Advantages: High resolution, fast separation, and small sample size; can be automated and integrated with detectors like UV or mass spectrometry.
Applications: Drug purity analysis, separation of nucleotides, peptides, and proteins, clinical diagnostics.
APPLICATIONS OF ELECTROPHORESIS:
1. Genetic Research: DNA and RNA electrophoresis are essential for genetic fingerprinting, cloning, and sequencing. Gel electrophoresis is used to check the size and purity of nucleic acids.
2. Protein Analysis: Protein electrophoresis (SDS-PAGE) is used to study protein structure, function, and molecular weight. Proteomics research uses electrophoresis for separating complex protein mixtures.
3. Clinical Diagnostics: Electrophoresis is employed in diagnosing diseases by separating and identifying serum proteins, hemoglobin variants, or analyzing lipoproteins.
4. Forensics: DNA electrophoresis is a crucial technique in forensic science for DNA profiling and matching biological samples in crime scene investigations.
5. Pharmaceutical and Drug Development: Capillary electrophoresis is used for drug purity analysis, determination of pharmacokinetics, and quality control in pharmaceutical industries.
6. Environmental Science: Used in the analysis of pollutants, toxins, and heavy metals in environmental samples by separating small molecules and ions.

