1. Introduction to Enzyme Biotechnology
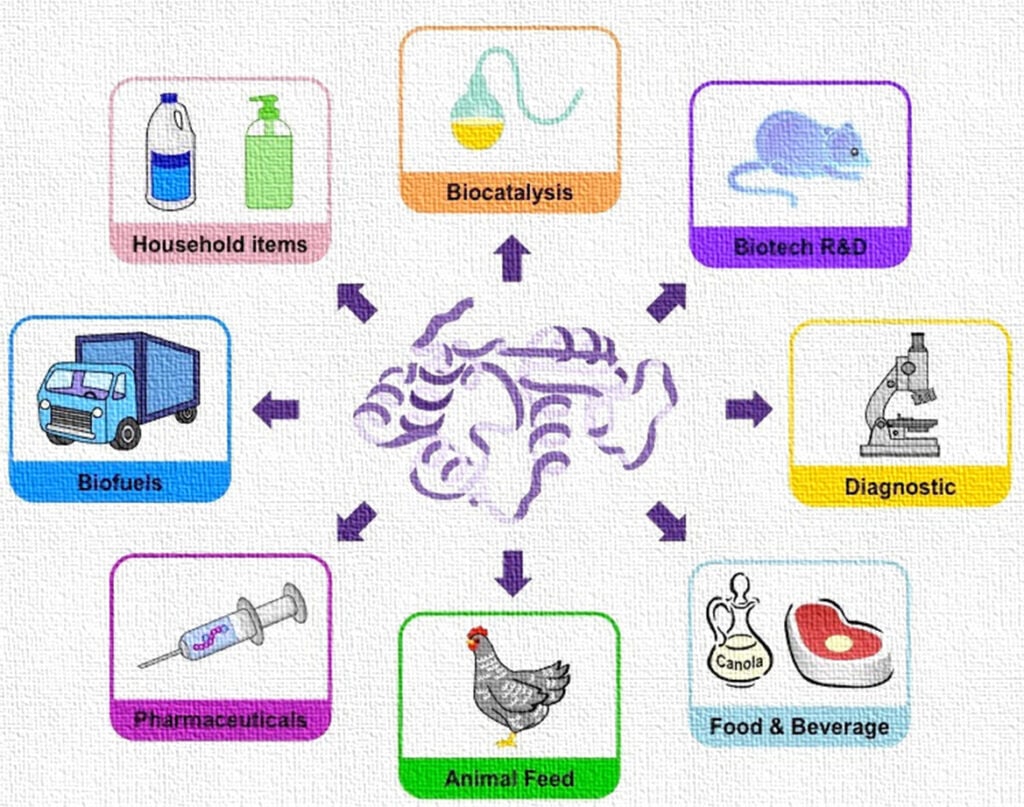
Enzyme biotechnology is a branch of biotechnology that focuses on the development and application of enzymes for industrial, medical, environmental, and research purposes. Enzymes are biocatalysts that accelerate chemical reactions with high specificity and efficiency under mild conditions of temperature and pH. The advent of enzyme biotechnology has revolutionized industries by making processes more sustainable, cost-effective, and environmentally friendly.
One of the critical challenges in enzyme technology is the reuse and stability of enzymes. Since free enzymes are soluble and often unstable under industrial conditions, immobilization techniques have been developed to enhance their operational stability, reusability, and control over catalytic activity. Immobilized enzymes play a pivotal role in continuous processing systems, biosensors, and bioreactors.
2. Enzyme Immobilization: Definition and Significance
2.1 What is Enzyme Immobilization?
Enzyme immobilization refers to the process of confining or anchoring enzyme molecules to a solid support or within a matrix, thereby restricting their mobility but retaining their catalytic activity.
2.2 Objectives of Immobilization
- Enhance enzyme stability and activity.
- Allow enzyme reuse in multiple reaction cycles.
- Facilitate separation from reaction media.
- Improve process control and product purity.
- Enable continuous operation in industrial processes.
3. Methods of Enzyme Immobilization
Immobilization techniques can be broadly categorized into physical and chemical methods, each with specific advantages and limitations.
3.1 Adsorption
Principle: Adsorption is the physical attachment of enzyme molecules onto the surface of carriers via weak forces such as van der Waals interactions, hydrophobic interactions, ionic bonds, and hydrogen bonds.
Carriers Used
- Porous glass
- Activated carbon
- Clays
- Synthetic resins
- Ion exchange resins
Advantages
- Simple and inexpensive.
- No chemical modification required.
- Minimal effect on enzyme structure and activity.
Limitations
- Weak binding may lead to enzyme leaching.
- Affected by changes in pH, ionic strength, and temperature.
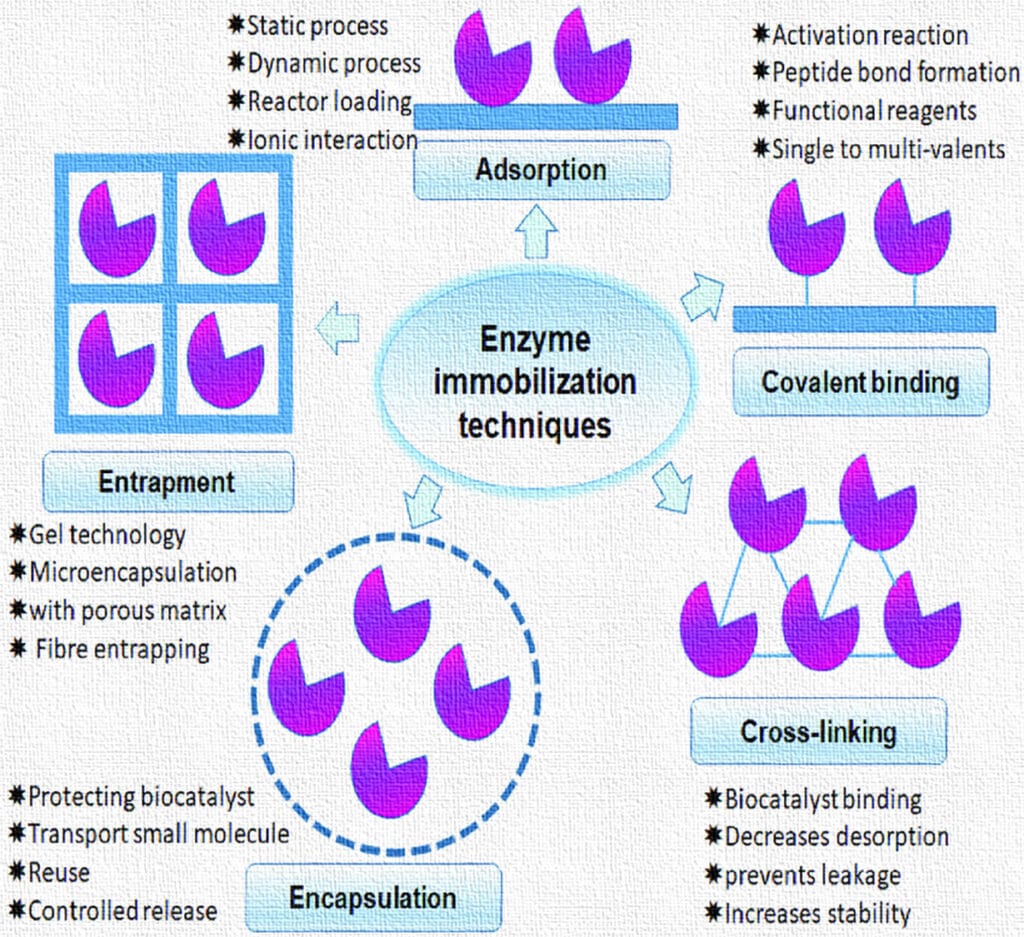
3.2 Covalent Bonding
Principle: Involves the formation of stable covalent bonds between enzyme functional groups (amino, carboxyl, thiol, hydroxyl) and functional groups on the support matrix.
Carriers Used
- Agarose
- Cellulose derivatives
- Silica
- Sepharose
- Polyacrylamide beads
Common Crosslinking Agents
- Glutaraldehyde
- Carbodiimides
- Cyanogen bromide (CNBr)
Advantages
- Strong and stable binding.
- Minimal enzyme leaching.
- Suitable for harsh operational conditions.
Limitations
- May involve loss of enzymatic activity due to modification of active site.
- Complex preparation.
3.3 Entrapment (Encapsulation)
Principle: Enzymes are physically confined within the pores of a gel or polymer matrix, without binding to the matrix. The substrate and products can diffuse in and out freely.
Matrices Used
- Alginate beads (calcium alginate)
- Polyacrylamide
- Carrageenan
- Gelatin
- Silica gel
Advantages
- Protects enzyme from harsh environments.
- Maintains high enzymatic activity.
- Easy to implement.
Limitations
- Limited diffusion of substrates/products.
- Possible enzyme leakage.
- Not suitable for large substrates.
3.4 Cross-Linking (Carrier-Free Immobilization)
Principle: Enzymes are cross-linked to form aggregates without a carrier. This method is also known as cross-linked enzyme aggregates (CLEAs).
Crosslinking Agents
- Glutaraldehyde
- Diisocyanates
Advantages
- High volumetric activity (no inert carrier).
- Economical and environmentally friendly.
- Stable under extreme conditions.
Limitations
- Diffusional limitations.
- Mechanical fragility.
3.5 Encapsulation (Microencapsulation)
Principle: Enzymes are enclosed within a semi-permeable membrane or capsule which allows passage of substrates and products but retains the enzyme.
Materials Used
- Polyamide membranes
- Liposomes
- Cellulose acetate
Advantages
- Offers protection from denaturation.
- Can be used in medical and biosensor applications.
Limitations
- Membrane may become fouled or clogged.
- Diffusion limitations.
4. Carriers Used for Immobilization
The selection of a carrier material is crucial for immobilization. An ideal support should:
- Be chemically inert and physically stable.
- Provide high surface area.
- Be compatible with the enzyme.
- Allow easy recovery and reuse.
Examples of Carriers:
| Type | Examples |
| Natural | Cellulose, agarose, alginate, chitosan |
| Synthetic | Polyacrylamide, polystyrene, silica |
| Inorganic | Zeolites, glass beads, activated carbon |
5. Factors Affecting Enzyme Immobilization
Several parameters influence the efficiency of enzyme immobilization:
- pH and temperature: Affect enzyme structure and binding efficiency.
- Ionic strength: Influences adsorption and electrostatic interactions.
- Enzyme loading: Excess enzyme may cause crowding and reduced activity.
- Matrix porosity: Affects diffusion and substrate accessibility.
- Binding time and conditions: Determine the extent of attachment and activity retention.
6. Characterization of Immobilized Enzymes
To evaluate the performance of immobilized enzymes, the following parameters are considered:
- Activity Retention (%): Comparison of immobilized vs. free enzyme.
- Stability: Thermal, pH, and storage stability.
- Reusability: Number of cycles with retained activity.
- Kinetic parameters: Km and Vmax changes post-immobilization.
- Mechanical strength and porosity: Important for bioreactor applications.
7. Applications of Immobilized Enzymes
7.1 Industrial Applications
1. Food Industry
- Lactase: Used to hydrolyze lactose in milk (for lactose-intolerant individuals).
- Invertase: Converts sucrose to glucose and fructose in confectionery.
- Pectinase and cellulase: Used in juice clarification.
2. Brewing and Wine Making
- Amylase and glucanase: Breakdown of starches and beta-glucans.
- Protease: Used for chill-proofing beer.
3. Detergent Industry
- Proteases, lipases, and amylases: Included in detergents to remove protein, fat, and starch stains.
4. Dairy Industry
- Rennin (chymosin): Immobilized for cheese production.
- Lipase: For flavor enhancement in cheese ripening.
7.2 Pharmaceutical and Medical Applications
1. Biosensors
- Glucose oxidase: Immobilized in glucose biosensors for diabetic patients.
- Urease: For detection of urea levels.
- Cholinesterase: Detection of pesticides and nerve agents.
2. Drug Manufacturing
- Penicillin acylase: For synthesis of semi-synthetic penicillins.
- L-asparaginase: Used in leukemia treatment; immobilization enhances stability.
3. Diagnostic Kits
- Enzymes like peroxidase and alkaline phosphatase are used in ELISA and immunoassays.
7.3 Environmental Applications
1. Wastewater Treatment
- Laccases and peroxidases: Degrade phenols and dyes.
- Urease and nitrilase: Remove nitrogenous wastes.
2. Bioremediation
- Organophosphate hydrolase: Degrades toxic pesticides.
- Dehalogenases: Detoxify halogenated compounds.
7.4 Biofuel Production
- Cellulase and hemicellulase: Break down lignocellulose into fermentable sugars.
- Lipase: Used in biodiesel production by transesterification.
7.5 Textile and Leather Industry
- Amylases and cellulases: Used for desizing and softening fabrics.
- Proteases: Remove hair and soften hides.
7.6 Paper and Pulp Industry
- Xylanase: Aids in bleaching of paper pulp.
- Laccase and peroxidase: Reduce lignin content in environmentally friendly ways.
8. Advantages of Enzyme Immobilization
- Operational Stability: Retains activity under extreme conditions.
- Reusability: Lowers cost by enabling multiple cycles.
- Continuous Processing: Facilitates automation and scalability.
- Reduced Contamination: Easy removal from product mixture.
- Enhanced Product Quality: More consistent and pure final products.
9. Limitations and Challenges
- Enzyme Inactivation: During or after immobilization.
- Mass Transfer Limitations: Due to restricted diffusion in porous supports.
- High Initial Cost: Some techniques require expensive materials and setup.
- Carrier Degradation: Over long-term use in industrial processes.
10. Recent Advances and Future Prospects
1. Nanotechnology in Enzyme Immobilization: Nanoparticles (e.g., gold, magnetic, silica) offer high surface area and facilitate easy recovery using magnets or centrifugation.
2. Genetic Engineering: Recombinant enzymes can be designed with tags (e.g., His-tags) for site-specific immobilization.
3. Smart Polymers: Responsive to pH, temperature, or light, enabling on-demand enzyme activation.
4. Microfluidics and Lab-on-Chip: Use immobilized enzymes for real-time, point-of-care diagnostics and assays.
5. 3D Printing: Creating customized bioreactor setups with embedded immobilized enzymes.
Conclusion
Enzyme immobilization is a cornerstone of enzyme biotechnology with widespread implications across diverse fields. It enhances enzyme performance, facilitates cost-effective production, and contributes to sustainable practices. Continuous innovation in materials science, nanotechnology, and bioengineering is expanding the potential of immobilized enzymes in industrial and therapeutic domains.
Future prospects indicate a shift toward smart, recyclable, and highly efficient immobilized systems that could revolutionize not only production lines but also personalized medicine, environmental restoration, and real-time diagnostics.

