Introduction
Estimating the concentration of magnesium sulfate: Magnesium sulfate (MgSO₄) is an inorganic salt containing magnesium, sulfur, and oxygen. It is widely used in pharmaceutical, agricultural, and industrial applications. Estimating the concentration of magnesium sulfate in a solution is essential in various fields, including medical formulations, laboratory analyses, and industrial quality control.
Several analytical techniques are available for determining the concentration of MgSO₄, including gravimetric analysis, titrimetric analysis, spectrophotometry, and atomic absorption spectroscopy (AAS).
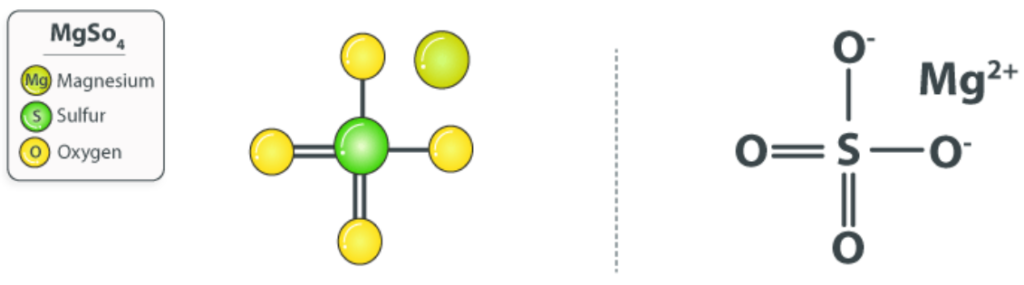
Methods for Estimating Magnesium Sulfate Concentration
1. Gravimetric Analysis
Preparation of Solution:
- A known volume of MgSO₄ solution is taken.
- The solution is acidified using hydrochloric acid (HCl) to prevent interference.
Precipitation of Magnesium as Magnesium Ammonium Phosphate:
- Ammonium phosphate ((NH₄)₂HPO₄) is added to the solution.
- The solution is heated to facilitate precipitation.
- Ammonia (NH₃) is added to maintain alkaline conditions, ensuring complete precipitation.
- The precipitate (MgNH₄PO₄·6H₂O) is filtered and washed
Conversion to Magnesium Pyrophosphate:
The precipitate is ignited at 1000°C to convert it into magnesium pyrophosphate (Mg₂P₂O₇).
Weighing and Calculation:
- The mass of the obtained Mg₂P₂O₇ is measured.
- The concentration of MgSO₄ is calculated using the formula:
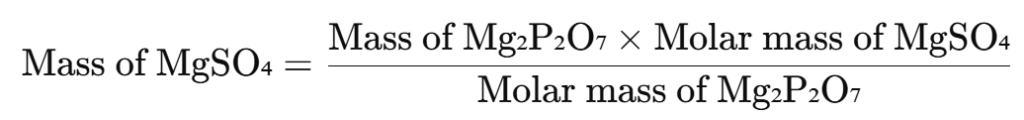
2. Complexometric Titration using EDTA
Ethylenediaminetetraacetic acid (EDTA) is a chelating agent that forms a stable complex with magnesium ions (Mg²⁺).
Procedure:
Preparation of Reagents:
- Prepare a standard EDTA solution.
- Buffer solution (pH ~10) is prepared using ammonium chloride-ammonia buffer.
- Eriochrome Black T (EBT) is used as an indicator, which forms a pink complex with Mg²⁺.
Titration Process:
- A known volume of MgSO₄ solution is taken in a conical flask.
- Add the buffer solution to maintain pH 10.
- Add a few drops of Eriochrome Black T indicator; the solution turns wine red.
- Titrate with EDTA solution until the color changes from red to blue, indicating the end point.
Calculation of MgSO₄ Concentration:
The concentration of MgSO₄ is determined using the titration equation:
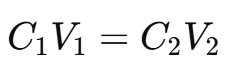
Where
C1 = Concentration of MgSO₄
V1 = Volume of MgSO₄ solution
C2 = Concentration of EDTA
V2 = Volume of EDTA used
3. Spectrophotometric Analysis
Spectrophotometry is used to determine Mg²⁺ concentration by forming a colored complex with specific reagents.
Procedure:
Preparation of Standard Solutions: Prepare a series of MgSO₄ standard solutions of known concentrations.
Reaction with Reagent: Add Titan Yellow dye or Calmagite reagent, which forms a colored complex with Mg²⁺.
Absorbance Measurement: Measure the absorbance of the solution at a specific wavelength (typically 530 nm for Titan Yellow). A calibration curve is plotted using the standard solutions.
Calculation:
The concentration of MgSO₄ in the unknown sample is determined from the calibration curve.
4. Atomic Absorption Spectroscopy (AAS)
Atomic absorption spectroscopy (AAS) is a highly accurate method for determining the Mg²⁺ concentration in a solution.
Procedure:
Preparation of Standards and Sample:
- Prepare MgSO₄ standard solutions.
- Dilute the unknown MgSO₄ solution as needed.
AAS Analysis:
- Use a flame atomic absorption spectrophotometer with a magnesium hollow cathode lamp.
- Measure the absorbance of the sample at 285.2 nm.
Concentration Determination:
Compare the sample absorbance with that of the standard solutions to determine the MgSO₄ concentration.
Comparison of Methods
| Method | Principle | Sensitivity | Accuracy | Complexity |
|---|---|---|---|---|
| Gravimetric Analysis | Precipitation of Mg²⁺ as Mg₂P₂O₇ | Moderate | High | High |
| EDTA Titration | Complexation with EDTA | Moderate | Moderate | Low |
| Spectrophotometry | Absorbance of Mg²⁺-reagent complex | High | High | Moderate |
| AAS | Absorption of light by Mg atoms | Very High | Very High | High |
The choice of method for estimating the concentration of magnesium sulfate depends on the required accuracy, available equipment, and sample type.
- Gravimetric analysis is highly accurate but time-consuming.
- EDTA titration is a simple and widely used technique in laboratories.
- Spectrophotometry is useful for lower concentrations and colored solutions.
- AAS provides the highest precision and sensitivity, ideal for trace analysis.
Each method has its applications in different fields such as pharmaceuticals, agriculture, and industrial analysis.
Visit to: Pharmacareerinsider.com

