Factors Affecting Fluorescence
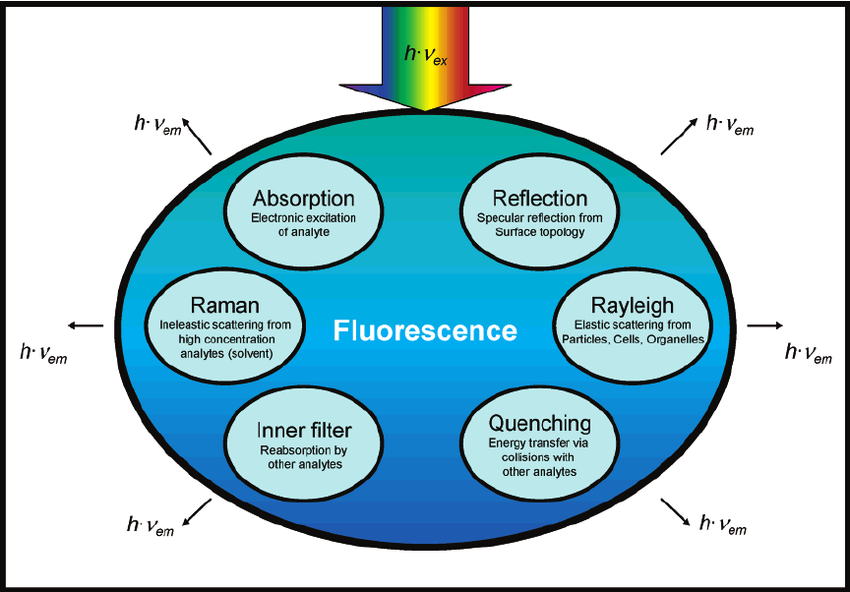
Factors Affecting Fluorescence: Fluorimetry is a highly sensitive and selective analytical technique widely utilized in the quantitative and qualitative analysis of fluorescent substances, including pharmaceutical compounds. This method relies on the ability of certain molecules to absorb light at a specific wavelength and then emit light at a longer wavelength. The intensity and wavelength of the emitted light depend on several intrinsic and extrinsic factors, making fluorescence-based analysis an essential tool in drug discovery, pharmaceutical quality control, and biomedical research. The fluorescence of a compound is influenced by various factors, including its molecular structure, environmental conditions, and instrumental parameters. Understanding these factors is crucial for optimizing fluorescence-based analytical techniques and ensuring accurate and reproducible results.
Suggested Post: Click here
Factors Affecting Fluorescence
Molecular Structure and Functional Groups
The intrinsic fluorescence of a molecule is primarily determined by its chemical structure. The presence of certain functional groups and conjugated systems can significantly influence the fluorescence intensity and emission wavelength. Some of the key structural factors include:
- Aromatic Rings: Compounds with conjugated π-electron systems, such as aromatic rings, exhibit strong fluorescence due to their ability to undergo electronic transitions upon light absorption. Examples of such molecules include benzene, naphthalene, anthracene, and their derivatives, which are known for their high fluorescence quantum yields.
- Heteroatoms and Electron-Donating Groups: Functional groups such as hydroxyl (-OH), amine (-NH2), and methoxy (-OCH3) enhance fluorescence by stabilizing the excited state, thereby increasing the probability of radiative decay (fluorescence emission).
- Electron-Withdrawing Groups: Groups such as nitro (-NO2) and carboxyl (-COOH) tend to quench fluorescence by increasing non-radiative decay pathways, reducing the overall fluorescence intensity of the compound.
Quantum Yield
Quantum yield (Φ) is an important parameter that describes the efficiency of fluorescence emission. It is defined as the ratio of emitted photons to absorbed photons. A high quantum yield indicates strong fluorescence and is desirable for analytical applications. The quantum yield is influenced by several factors:
- Non-Radiative Decay: If a molecule undergoes internal conversion or intersystem crossing, the energy is dissipated non-radiatively rather than being emitted as fluorescence, resulting in a lower quantum yield.
- Radiative Decay: A high radiative decay rate, where absorbed energy is efficiently converted into emitted fluorescence, leads to a higher quantum yield and better fluorescence intensity.
Solvent Effects
The choice of solvent plays a critical role in fluorescence studies, as it affects the polarity, hydrogen bonding, and potential quenching of fluorescence emission.
- Polarity: Polar solvents can stabilize excited states, leading to shifts in fluorescence spectra (either redshift or blueshift, depending on the nature of the fluorophore). Nonpolar solvents, on the other hand, may preserve the intrinsic fluorescence properties of the compound.
- Hydrogen Bonding: Solvent molecules capable of hydrogen bonding can interact with the fluorophore and cause fluorescence quenching by increasing non-radiative decay pathways. This effect is commonly observed in protic solvents like water and alcohols.
- Solvent Viscosity: High-viscosity solvents restrict molecular motion, reducing internal conversion and enhancing fluorescence intensity by limiting non-radiative energy loss.
pH and Ionic Strength
The pH and ionic strength of the medium can significantly impact fluorescence intensity, particularly for compounds that contain ionizable functional groups.
- pH Dependence: Many fluorophores exhibit pH-dependent fluorescence due to protonation or deprotonation of functional groups. For example, fluorescein shows a pH-dependent shift in fluorescence intensity, with higher fluorescence in alkaline conditions.
- Ionic Strength Effects: High salt concentrations can lead to fluorescence quenching by disturbing the excited-state equilibrium of the fluorophore. Certain metal ions can also interact with fluorophores, either enhancing or quenching fluorescence depending on their specific interactions.
Temperature
Temperature is an important factor affecting fluorescence, as it influences molecular motion and energy dissipation processes.
- Fluorescence Intensity and Temperature: Generally, fluorescence intensity decreases with increasing temperature due to enhanced molecular collisions, which promote non-radiative decay mechanisms such as internal conversion and vibrational relaxation.
- Lower Temperatures Enhance Fluorescence: At lower temperatures, vibrational relaxation is minimized, leading to stronger fluorescence signals. This principle is often utilized in low-temperature fluorescence studies to obtain high-resolution spectra.
Oxygen and Other Quenchers
Molecular oxygen is a well-known quencher of fluorescence, as it facilitates intersystem crossing to the triplet state, leading to energy dissipation without photon emission. Other quenching mechanisms include:
- Collisional Quenching: This occurs when fluorophores undergo transient interactions with quenching agents such as halide ions (I-, Br-, Cl-) and heavy metals, leading to non-radiative energy loss.
- Static Quenching: Some fluorophores form stable, non-fluorescent complexes with quenchers, reducing fluorescence intensity.
Excitation and Emission Wavelengths
Fluorescence intensity is dependent on the excitation wavelength, and choosing the optimal excitation wavelength ensures maximum emission intensity.
- Stokes Shift: The difference between excitation and emission wavelengths, known as the Stokes shift, is important for minimizing self-absorption and improving detection sensitivity in fluorescence studies.
- Optimal Wavelength Selection: Excitation at the peak absorption wavelength of the fluorophore maximizes fluorescence output, leading to better analytical sensitivity.
Concentration of Fluorophores
At low concentrations, fluorescence intensity follows a linear relationship with concentration, as described by the Beer-Lambert law. However, at high concentrations, fluorescence quenching can occur due to inner filter effects and self-quenching phenomena.
Instrumental Factors
- Light Source: The intensity and stability of the excitation source (e.g., xenon lamp, LED, laser) directly affect fluorescence measurements.
- Detector Sensitivity: High-sensitivity detectors, such as photomultiplier tubes (PMTs), improve fluorescence detection.
- Slit Width and Monochromators: These parameters control the amount of light reaching the detector, influencing signal-to-noise ratio and resolution.
Characteristics of Drugs Analyzed by Fluorimetry
Drugs that exhibit intrinsic fluorescence or can be derivatized to produce fluorescent derivatives are suitable for fluorimetric analysis. Fluorimetry is widely used in pharmaceutical analysis due to its high sensitivity and selectivity.
Structural Features Favoring Fluorescence
- Polycyclic Aromatic Systems: Drugs with extended conjugation, such as anthracyclines, exhibit strong fluorescence.
- Planarity and Rigidity: Planar molecules with rigid structures reduce non-radiative decay, enhancing fluorescence efficiency.
Drug Categories Suitable for Fluorimetry
- Antibiotics: Tetracyclines and fluoroquinolones exhibit strong fluorescence.
- Vitamins: Riboflavin (B2) and folic acid fluoresce under specific conditions.
- Alkaloids: Quinine, morphine, and codeine exhibit fluorescence.
- Anti-Cancer Drugs: Doxorubicin and daunorubicin are fluorescent anthracyclines.
- Steroids and Hormones: Estrogens and corticosteroids can be analyzed using fluorimetry.
Conclusion
Fluorimetry is a highly effective technique for drug analysis, influenced by molecular, environmental, and instrumental factors. Many pharmaceutical compounds exhibit intrinsic fluorescence or can be derivatized, making fluorimetry an essential tool in pharmaceutical and biomedical research.



