Introduction
Flame Emission Spectroscopy (FES) is an analytical technique extensively used to determine the concentration of specific metal ions by analyzing the light emitted when these atoms are excited in a flame. This method is particularly effective for detecting alkali and alkaline earth metals due to their strong emission spectra. The technique is widely applied in clinical diagnostics, pharmaceutical analysis, environmental monitoring, and industrial quality control due to its simplicity, cost-effectiveness, and rapid detection capabilities.
Suggested post: UV Visible Spectroscopy
Principle of Flame Emission Spectroscopy
FES is founded on the principle that when a sample containing metal ions is introduced into a flame, the thermal energy from the flame excites the atoms, elevating their electrons to higher energy levels. As these electrons return to their ground state, they emit light at characteristic wavelengths unique to each element. The emitted light is then measured to determine the concentration of the specific element in the sample. The fundamental steps involved in FES include:
- Nebulization: The sample, typically in liquid form, is introduced into the flame as a fine mist using a nebulizer.
- Desolvation and Vaporization: The solvent in the sample evaporates, leaving behind solid particles that subsequently vaporize into free atoms.
- Excitation: The high temperature of the flame provides sufficient energy to excite the atoms, causing their electrons to jump to higher energy states.
- Emission of Light: When these excited electrons return to their original energy levels, they release energy in the form of light, with specific wavelengths corresponding to the element.
- Detection and Measurement: The emitted light is analyzed using optical components such as monochromators and photodetectors to quantify the element present in the sample.
Since the intensity of the emitted light is directly proportional to the element’s concentration, FES serves as an effective quantitative analytical technique.
Instrumentation of Flame Emission Spectroscopy
The essential components of a Flame Emission Spectroscopy system include:
1. Flame Source: The flame serves as the excitation source, providing the thermal energy required to excite atoms. The type of flame used depends on the element under analysis and the required sensitivity. Common fuel-oxidant combinations include:
- Air-acetylene: Produces a flame temperature of approximately 2000-2500°C, commonly used for alkali metals.
- Nitrous oxide-acetylene: Generates a higher temperature of around 2600-3000°C, suitable for elements requiring more energy for excitation.
- Hydrogen-oxygen: Yields an even higher temperature of about 2800-3000°C, useful for refractory elements.
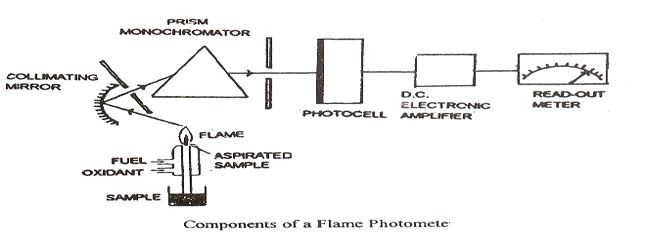
2. Sample Introduction System: A nebulizer converts the liquid sample into a fine aerosol that can be efficiently introduced into the flame.A spray chamber assists in producing a uniform mist, ensuring a consistent sample delivery to the flame.
3. Monochromator: This optical component isolates the specific wavelength of light emitted by the excited atoms to minimize interference from other wavelengths.Types of monochromators include prisms and diffraction gratings, which selectively filter the emitted radiation.
4. Detector: Photomultiplier tubes (PMTs) or photodiodes are used to detect the emitted light.The detected signal is converted into an electrical output proportional to the intensity of the emitted radiation.
5. Readout System: The processed signal is displayed in an understandable format, such as a digital readout in parts per million (ppm) or parts per billion (ppb).
Interferences in Flame Emission Spectroscopy
Several factors can influence the accuracy and reliability of FES measurements. These include:
1. Spectral Interference: Occurs when emission lines of different elements overlap, leading to misinterpretation of results.Can be mitigated using high-resolution monochromators or interference filters to enhance spectral selectivity.
2. Chemical Interference: Happens when chemical reactions in the flame inhibit atomization.
Example: Phosphate ions can suppress calcium emission, leading to underestimation. Can be corrected by adding releasing agents or using higher flame temperatures.
3. Ionization Interference: Occurs when atoms become ionized instead of remaining in their neutral excited state.Common in alkali metals at high flame temperatures.Minimized by adding an excess of a more easily ionizable element, such as potassium for sodium analysis.
4. Physical Interference: Caused by variations in sample viscosity, surface tension, or density, affecting sample nebulization and atomization.Reduced by matching sample matrices or using internal standards for calibration.
Applications of Flame Emission Spectroscopy
Due to its simplicity and efficiency, FES is widely applied in numerous fields:
1. Clinical and Medical Applications: Used for the determination of sodium (Na) and potassium (K) levels in biological fluids such as serum, urine, and plasma.Plays a crucial role in diagnosing electrolyte imbalances, which are essential in monitoring kidney function, cardiovascular health, and metabolic disorders.
2. Pharmaceutical Analysis: Used for detecting metal impurities in pharmaceutical formulations.Ensures quality control of drug products containing alkali and alkaline earth metals.
3. Environmental Monitoring: Analyzes metal contamination in water, soil, and air samples.Detects toxic metals in industrial effluents and waste management systems.
4. Agricultural and Food Analysis: Determines the presence of essential minerals in soil and fertilizers.Measures sodium and potassium levels in food products for nutritional analysis.
5. Industrial Applications: Used in metal refining industries for quality control.Determines metal content in fuels, lubricants, and alloys.
6. Forensic and Toxicological Studies: Identifies toxic metal exposure in forensic investigations.Detects metal poisoning in biological samples, aiding in criminal investigations.
Advantages of Flame Emission Spectroscopy
- High Sensitivity: Capable of detecting trace amounts of elements.
- Rapid Analysis: Provides quick and efficient results with minimal sample preparation.
- Cost-Effective: Less expensive compared to other spectroscopic methods.
- Selective for Alkali and Alkaline Earth Metals: Especially effective for these elements, ensuring precise determination.
Limitations of Flame Emission Spectroscopy
- Restricted to Group I and II Elements: Ineffective for non-metals and transition metals.
- Limited Sensitivity for Some Elements: Elements with low excitation efficiency may not be accurately detected.
- Flame Instability: Variations in flame conditions can impact measurement accuracy.
- Interferences: Requires careful handling of spectral, chemical, and physical interferences.
Conclusion
Flame Emission Spectroscopy remains an invaluable analytical technique for detecting and quantifying metal ions in various fields. Its affordability, simplicity, and high sensitivity for alkali and alkaline earth metals make it indispensable in clinical, environmental, and industrial applications. Despite its limitations, proper methodological adjustments can significantly improve accuracy and reliability, ensuring its continued significance in elemental analysis.

