Introduction to Fractional Distillation
Fractional distillation is an advanced separation technique that refines the principles of simple distillation. It is employed to separate components in a liquid mixture based on their boiling points by utilizing a fractionating column, which enhances the separation efficiency. This process is crucial in the production of high-purity substances and is commonly used in industries such as petrochemicals, pharmaceuticals, and the production of alcoholic beverages.
Basic Principles of Fractional Distillation
a. Vapor-Liquid Equilibrium:
– Fractional distillation relies on achieving multiple vaporization-condensation cycles, enhancing the separation of components based on their boiling points.
b. Fractionating Column:
– The fractionating column, packed with materials that provide surfaces for vaporization and condensation, allows for repeated vapor-liquid equilibrium.
c. Temperature Gradients:
– The column establishes temperature gradients, with higher temperatures at the bottom and lower temperatures at the top, creating distinct separation zones.
d. Enrichment of Vapor:
– As vapor rises through the column, it becomes enriched in the more volatile components.
e. Multiple Condensation:
– The vapor, rich in the more volatile component, condenses and is partially returned to the column, contributing to multiple cycles.
Methodology of Fractional Distillation
a. Apparatus
1. Distillation Flask:
Contains the liquid mixture to be distilled.
2. Fractionating Column:
A tall column with a series of trays or packing materials to create vaporization-condensation cycles.
3. Condenser:
Converts vapor back into liquid distillate.
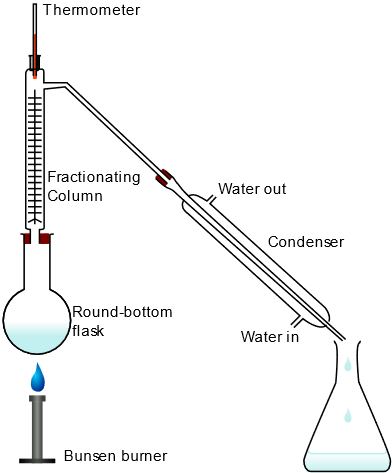
4. Receiver Flask:
Collects the separated components.
5. Heating Source:
Provides heat to the distillation flask.
b. Procedure
1. Loading the Flask:
The liquid mixture is placed in the distillation flask.
2. Heating:
The flask is heated, and vapor rises into the fractionating column.
3. Vaporization:
As the vapor ascends through the column, it undergoes repeated vaporization-condensation cycles.
4. Condensation:
The vapor rich in the more volatile component condenses and is partially returned to the column.
5. Collection:
The condensed liquid (distillate) is collected in the receiver flask.
6. Temperature Monitoring:
Temperature is monitored, and fractions are collected at different temperature ranges.
7. Analysis of Fractions:
Fractions collected at different temperatures represent different components of the mixture.
c. Factors Influencing Fractional Distillation
1. Column Efficiency:
The efficiency of the fractionating column is crucial for effective separation.
2. Boiling Point Difference:
A greater difference in boiling points enhances separation.
3. Heat Source Control:
Controlling the heat source prevents excessive bumping or boiling over.
4. Uses
1. Petroleum Refining:
Fractional distillation is a key process in refining crude oil into various petroleum products.
2. Chemical Industry:
Applied in the separation and purification of chemicals and solvents.
3. Alcohol Production:
Used in the production of alcoholic beverages to separate ethanol from other components.
4. Pharmaceuticals:
Fractional distillation is crucial for the production of high-purity pharmaceutical compounds.
Merits of Fractional Distillation
1. High Separation Efficiency:
Fractional distillation offers higher separation efficiency compared to simple distillation.
2. Precise Component Isolation:
Enables the isolation of specific components in a mixture.
3. Versatility:
Can be applied to a wide range of mixtures with varying boiling points.
Demerits of Fractional Distillation
1. Complexity:
Fractional distillation is more complex and requires additional equipment compared to simple distillation.
2. Energy Consumption:
The process can be energy-intensive, especially for large-scale operations.
3. Initial Cost:
The initial installation cost is higher compared to simple distillation setups.
Fractional distillation is a sophisticated separation technique that builds upon the principles of simple distillation. Its use of a fractionating column enhances separation efficiency, making it indispensable in various industries. Understanding the methodology and optimizing the factors influencing the process are crucial for achieving precise and efficient separation of components in a liquid mixture.

