The gaseous state is the simplest among the three states of matter. In a gaseous state, molecules within the container move in any direction as they are widely spaced in space. These molecules exert pressure on the container walls in all directions. Gases can expand indefinitely to fill the entire container. When a movable piston applies pressure, gases can easily compress. When different gases are placed together, they rapidly diffuse to form a homogeneous mixture. Heating a gas in a container increases its pressure, and if a piston is present, the volume of the gas increases under these conditions.
Chemical properties of gases exhibit significant variation, while physical properties are more straightforward. Examining the individual actions of molecules on a small scale or the collective behavior of the gas on a larger scale explains the gaseous state. Studying these properties helps comprehend the behavior of gases, and the kinetic molecular theory provides a model that effectively describes these properties.
Kinetic molecular theory of ideal gases
The Kinetic Molecular Theory applies specifically to ideal gases, providing a qualitative explanation for their behavior. The key postulates include:
1. All matter comprises tiny particles (molecules or atoms).
2. Small particles that are widely spaced constitute ideal gases.
3. These particles are dimensionless points with zero volume.
4. They follow well-defined laws and are in rapid, random, and constant straight-line motion.
5. No attractive forces exist between gas molecules or between molecules and the container.
6. Molecules collide with each other and with the container walls.
7. Energy is transferred during collisions.
8. Collisions conserve energy, with potential energy shifting between molecules.
9. Energy distribution follows the Maxwell-Boltzmann Distribution.
10. Molecules in a gas sample possess varying energy levels; the average kinetic energy is proportional to the absolute temperature.
These postulates apply primarily to ideal gases and provide an approximate description of real gases.
Characteristics of gases
The gas in the container exhibits measurable characteristic properties such as volume (V), pressure (P), temperature (T), and the number of moles (n).
Volume:
The volume of the gas sample, typically measured in units of liters (L) or milliliters (mL), represents the container volume.
Pressure:
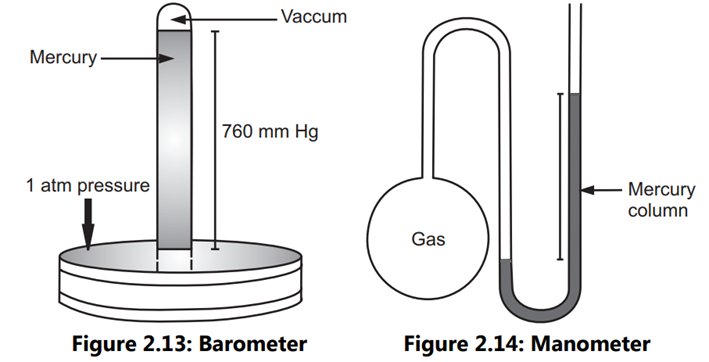
A barometer (Fig. 2.13) gauges atmospheric pressure. When a mercury-filled tube is inverted into a dish of mercury, the mercury flows until its column’s pressure equals the atmospheric pressure on the mercury’s surface. The height of the mercury column is 760 mm for 1 atm of pressure. In a manometer (Fig. 2.14), the height ‘h’ of the mercury column indicates the pressure difference between the gas inside the container and the external atmosphere.
Gas pressure is proportional to the average force per unit area exerted by gas molecules on the container walls. An increased number of gas molecules leads to higher pressure due to a greater frequency of collisions with the container walls. Reducing the container volume increases collisions, thereby raising pressure. Additionally, pressure is directly proportional to the kinetic energy of gas molecules, making higher temperatures correspond to greater kinetic energy and increased gas pressure.

