Aromatic Compounds have played a significant role in the progress of mankind, with ongoing potential for further contributions. They are integral to the composition of computer parts, DVDs, and crucial components in automotive systems. Commonly used drugs like Aspirin and paracetamol belong to the category of Aromatic Compounds. The field of organic chemistry specifically deals with Aromatic Compounds. Notably, Aromatic molecules demonstrate stability and do not readily react with other compounds. Aromaticity, a key property of Aromatic Compounds, is determined by Huckel’s Rule. Before delving into Huckel’s Rule, a thorough understanding of Aromaticity in organic chemistry is essential. It refers to the property of a cyclic, planar molecule with a resonance ring exhibiting greater stability than alternative geometric arrangements with the same atoms. To be an Aromatic Compound or possess Aromaticity, Compounds must fulfill the following four conditions –
- The molecule must be cyclic.
- Each atom within the cyclic ring must be part of a conjugated system, facilitating a delocalized pi-electron arrangement in the cyclic ring. Consequently, each atom in the cyclic ring must possess an empty p orbital, enabling active participation in resonance.
- All Compounds must follow Huckel’s Rule.
• The molecule must exhibit planarity or flatness. Compounds adhering to the aforementioned four rules of aromaticity are typically flat, indicating the presence of sufficiently high potential energy in such conditions.
Thus, Huckel’s Rule is one of the criteria Aromatic Compounds should fulfill to possess Aromaticity. Now, let us understand what Huckel’s Rule is.
What is Huckel`s Rule?
According to Huckel’s Rule, all planar Aromatic Compounds must have 4n+2 pi-electrons where n is an integer (i.e., n 0, 1, 2, 3, 4…etc.). This Rule estimates whether a planar ring Compound will possess Aromatic properties.
In 1931, German physicist and chemist Erich Huckel introduced a theory to identify aromatic properties in planar ring molecules. Huckel formulated Huckel’s Rule, asserting that a cyclic, planar molecule is deemed aromatic if it possesses 4n + 2π electrons.
Huckel’s Rule comprises a set of algorithms that assess whether a molecule is aromatic, antiaromatic, or nonaromatic by considering both the number of π electrons and the physical structure of the ring system.
The following algorithm determines the number of π electrons in the case of an aromatic system.
N= 4n + 2
where n is an integer
The following algorithm is used to determine the number of π electrons in the case of an antiaromatic system.
N= 4n
where n is an integer
A Compound is Non-aromatic when it does not have a continuous ring of the conjugated p orbital in a planar conformation.
Let’s illustrate this with the examples of Benzene and Cyclo octa-tetraene, both featuring a ring structure. Despite both compounds having a ring, one displays aromaticity while the other does not. Determining which one possesses aromaticity can be accomplished through Huckel’s Rule. Examining the structure of Benzene it reveals 6 pi – electrons. By applying the formula 4n + 2 and setting n = 1, we get (4×1) + 2 = 6, confirming that it adheres to Huckel’s Rule. Thus, Benzene is classified as an Aromatic Compound, demonstrating aromaticity.
n = 1
(4×1) + 2(4×1)+2 = 6π electrons
Aromatic Compound
In contrast, when examining the structure of Cyclooctatetraene, as depicted below, it is evident that it contains 8 pi – electrons. Applying the formula 4n + 2 and substituting any integer for n results in (4n) + 2 ≠ 8. Consequently, it deviates from Huckel’s Rule. Cyclooctatetraene is deemed a non-aromatic compound and does not exhibit aromaticity.
(Number of pi- electrons = 8 as it has 4 pi- bonds, So, for any value of ‘n’, 4n+2 cannot be equal to 8.)
Why 4n + 2 Electrons?
Huckel’s Molecular Orbital theory asserts that a compound is considered particularly stable when it fills all its bonding molecular orbitals with paired electrons. This theory holds true for aromatic compounds, given their exceptional stability. In aromatic compounds, no orbitals are filled, and there is an absence of occupied anti-bonding orbitals. The lowest energy molecular orbital accommodates only 2 electrons, and each subsequent energy level, denoted by n, fills with 4 electrons, resulting in a total of 4n + 2π electrons.
A few Examples of Huckel’s Rule
- Cyclopropenyl Anion
4π Electrons having a planer conjugated system (4n,n=1)
2 Electrons- one double bond
2 Electrons- lone pair of carbon with a negative charge
Therefore, it is anti-aromatic.
- Cyclopropenyl Cation
2π with a planer conjugated system(neither 4n nor 4n+2)
2 Electrons-one double bond
Therefore, it is Non-Aromatic
- Naphthalene
10π Electrons with planer conjugated system (4n+2,n=2)
10 Electrons- five double bond
Therefore, it is Aromatic.
- Pentalene
8π Electrons with planer conjugated system (4n,n=2)
8 Electrons- four double bonds
Therefore, it is AntiAromatic.
- Furan
6π Electrons with planer conjugated system (4n+2,n=1)
4 Electrons- two double bonds
2 Electrons- lone pair of O
Therefore, it is Aromatic.
- Pyrrole
6π Electrons with planer conjugated system (4n+2,n=1)
4 Electrons- double bonds
2 Electrons- lone pair of N
- Pyridine
6π Electrons with planer conjugated system (4n+2,n=1)
6 Electrons- 3 double bonds
0 Electrons-lone pair of N as it is associated with a double bond.
Applications of Huckel’s Rule
Some other applications of Huckel’s Rule are listed below-

Thus, thiophene has 6 pi – electrons and follows Huckel’s Rule as 41+241+2 = 6. It also obeys all other conditions of Aromaticity and is an Aromatic Compound.
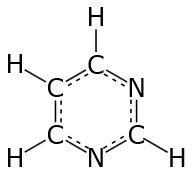
Thus, pyrimidine has 6 pi – electrons, and if we keep n= 1 in Huckel’s 4n + 2 Rule, then 41+241+2 = 6. So, pyrimidine follows Huckel’s Rule and fulfills other conditions of Aromaticity as well. Pyrimidine is an Aromatic Compound.

Naphthalene has 5 pi – bonds, containing 10 pi – electrons. If we keep n= 2 in the Huckel’s 4n + 2 Rule, then 42 + 2 = 10. Thus, it follows Huckel’s Rule and fulfills other conditions of Aromaticity as well. Naphthalene is an Aromatic Compound.

Thus, oxazole has 6 pi – electrons, and if we keep n= 1 in Huckel’s 4n + 2 Rule, then 41+241+2 = 6. So, oxazole follows Huckel’s Rule and fulfills other conditions of Aromaticity as well. Oxazole is an Aromatic Compound.

Thus, imidazole has 6 pi – electrons, and if we keep n= 1 in Huckel’s 4n + 2 Rule, then 41+241+2 = 6. So, imidazole follows Huckel’s Rule and fulfills other conditions of Aromaticity as well. Imidazole is an Aromatic Compound.
Exceptions to Huckel’s Rule
1. Cyclobutadiene C4H4 exhibits stability below 35 K, in contrast to the typical stability of planar ring molecules with 4n at higher temperatures.
2. Certain compounds, like pyrene, a polycyclic aromatic compound, deviate from Huckel’s Rule.
3. Trans-silicene, despite having 8 π bonds and being an aromatic polycyclic compound, does not adhere to Huckel’s Rule.

