Ideal solutions are a special class of mixtures in which the behavior of the mixture is predictable based on the idealized behavior of its components. In an ideal solution, the interactions between the molecules of different components are similar to the interactions between molecules of the same component. This leads to the absence of any significant deviations from Raoult’s law.
Key Characteristics of Ideal Solutions
1. Raoult’s Law: Raoult’s law describes the vapor pressure of an ideal solution. According to Raoult’s law, the partial vapor pressure of each component in the solution is proportional to its mole fraction in the mixture.
Mathematical Expression: For a binary mixture with components A and B:
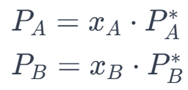
2. Zero Deviation from Ideality: In ideal solutions, there is no deviation from Raoult’s law. This means that the experimental vapor pressure of the mixture is the same as that predicted by Raoult’s law.
3. Enthalpy of Mixing: The enthalpy of mixing for ideal solutions is usually close to zero. This implies that there is no significant heat absorption or release upon mixing.
4. Volume Changes: Mixing ideal solutions does not result in any significant volume change.
5. Components: Ideal solutions are often observed in solutions of similar substances, such as two similar liquids or gases.
6. Temperature Dependence: The ideal behavior is more likely to be observed at higher temperatures.
It’s important to note that truly ideal solutions are rare in reality, as most solutions deviate to some extent from ideal behavior. However, ideal solutions serve as a useful theoretical concept for understanding the behavior of mixtures and for comparison with real solutions.

