Introduction
Ion Exchange Chromatography (IEC) is a powerful and versatile separation technique primarily used to purify proteins, peptides, amino acids, and other charged biomolecules based on their ionic charge. It separates molecules by their affinity to charged ion-exchange resins in the stationary phase. This technique is widely used in the pharmaceutical industry, biochemical research, water treatment, and environmental analysis.
Principle of Ion-Exchange Chromatography
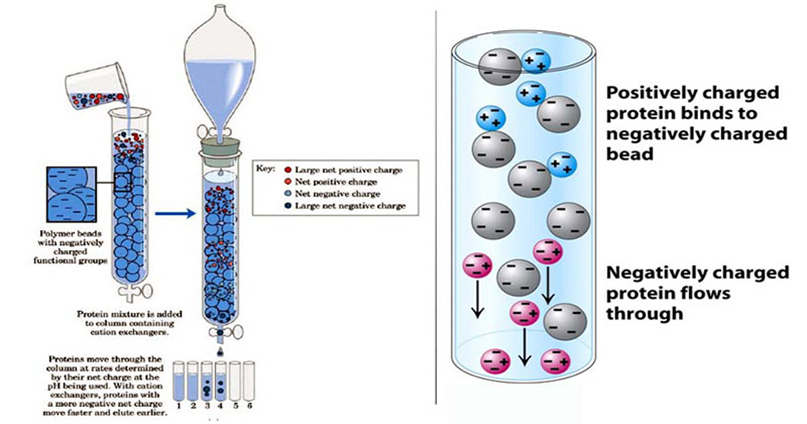
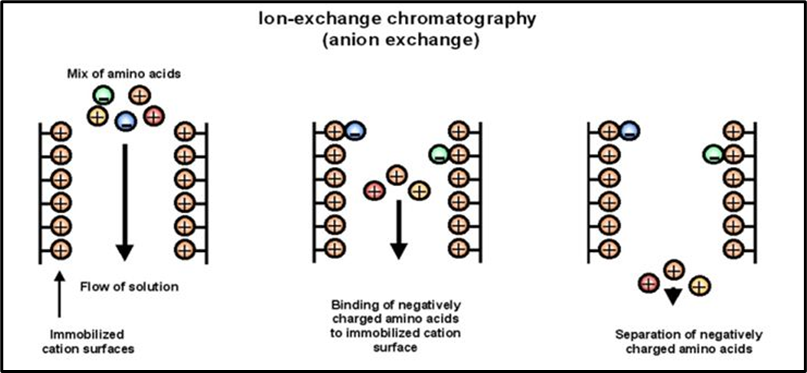
Ion-exchange chromatography works on the principle of attraction between charged resins and oppositely charged analytes. The process involves the exchange of charged ions (either positive or negative) to separate molecules based on their charge. In this technique, the stationary phase (resin) is coated with specific charged groups, which bind to the oppositely charged components in the sample mixture.
A cation or anion exchange resin, depending on the charge of the target molecules, binds these components by displacing the previously attached ions. The bound complexes are then eluted by using different buffers that alter the pH or ionic strength, allowing the components to be separated.
Classification of Ion Exchange Chromatography
Ion exchange chromatography can be broadly classified into two types based on the nature of ions being exchanged:
- Cation Exchange Chromatography: This involves the exchange of positively charged ions (cations) between the sample and the resin. The stationary phase in cation exchange contains negatively charged functional groups that bind to cations. Common cation exchange resins include those with carboxyl (-COO⁻) or sulfonate (-SO₃⁻) groups.
- Anion Exchange Chromatography: This involves the exchange of negatively charged ions (anions) between the sample and the resin. The stationary phase in anion exchange contains positively charged functional groups that bind to anions. Common anion exchange resins include those with amino (-NH₃⁺) or quaternary ammonium groups.
Ion Exchange Resins:
Ion exchange resins are the materials used in IEC that facilitate the exchange of ions between the sample and the stationary phase. They are usually composed of a polymer matrix with charged functional groups.
Types of Ion Exchange Resins:
- Strong Acidic Cation Exchangers: Have sulfonic acid (-SO₃H) groups and remain ionized across a wide pH range.
- Weak Acidic Cation Exchangers: Have carboxylic acid (-COOH) groups, ionized at higher pH levels.
- Strong Basic Anion Exchangers: Have quaternary ammonium groups, which are positively charged over a wide pH range.
- Weak Basic Anion Exchangers: Have primary, secondary, or tertiary amine groups and are ionized in lower pH conditions.
Properties of Ion Exchange Resins:
- Porosity: Determines the surface area available for ion exchange.
- Capacity: The number of ionic exchange sites available on the resin.
- Selectivity: The preference of the resin for specific ions over others.
- Swelling: Resins swell in water or buffer, which affects the flow and capacity.
- Stability: Resins must be chemically stable across a range of temperatures and pH levels.
Mechanism of Ion Exchange Process:
The ion exchange process relies on electrostatic interactions between the charged ions in the mobile phase and the oppositely charged functional groups on the resin in the stationary phase. The process involves the following steps:
- Equilibration: The resin is first equilibrated with a buffer to ensure that the charged groups on the resin are in their ionized form.
- Sample Application: The sample is introduced into the column, and charged analytes bind to the oppositely charged sites on the resin.
- Washing: Unbound molecules are washed out of the column using a buffer solution.
- Elution: Bound ions are displaced from the resin using an elution buffer containing competing ions or by changing the pH or ionic strength of the buffer, thereby separating different ions based on their affinity for the resin.
Factors Affecting Ion Exchange
Several factors influence the efficiency and resolution of ion exchange chromatography:
- pH: pH affects the ionization of both the analytes and the resin, influencing binding affinity. For example, proteins with a higher Isoelectric Point bind strongly in a cation exchange column under low pH.
- Ionic Strength: Increasing the salt concentration (ionic strength) in the buffer can displace weakly bound ions, promoting elution. High ionic strength buffers reduce ion binding capacity.
- Type of Resin: Different resins have varied charge densities and selectivity, which influence the resolution of separation.
- Flow Rate: A slower flow rate increases contact time between the resin and the analyte, improving separation but increasing the run time.
- Temperature: The ion exchange process is generally temperature-dependent. Higher temperatures can increase diffusion rates but may destabilize some biomolecules.
Instrumentation of ion exchange chromatography:
Typical ion exchange chromatography instrumentation includes: pump, injector, column, suppressor, detector, and recorder or data system.
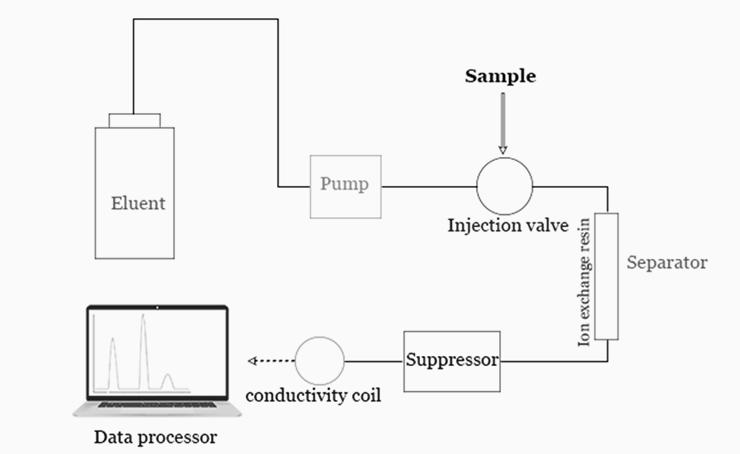
- Pump: The IC pump is considered to be one of the most important components in the system which has to provide a continuous constant flow of the eluent through the IC injector, column, and detector.
- Injector: Sample introduction can be accomplished in various ways. The simplest method is to use an injection valve. Liquid samples may be injected directly and solid samples need only to be dissolved in an appropriate solvent. Injectors should provide the possibility of injecting the liquid sample within the range of 0.1 to 100 ml of volume with high reproducibility and under high pressure (up to the 4000 psi).
- Columns: Depending on the application, ion exchange chromatography columns can be made from materials like stainless steel, titanium, glass, or inert plastics such as PEEK. Their diameters range from 2 mm to 5 cm, and lengths from 3 cm to 50 cm, suitable for various analytical purposes, including microanalysis, high-speed analyses, or preparative work. A guard column is placed before the separation column to protect it and extend its lifespan by filtering out particles that could clog the main column.
- Suppressor: The suppressor reduces the background conductivity of the chemicals used to elute samples from the ion-exchange column which improves the conductivity measurement of the ions being tested. IC suppressors are membrane-based devices which are designed to convert the ionic eluent to water as a means of enhancing the sensitivity.
- Detectors: Electrical conductivity detector is commonly use.
- Data system: In routine analysis, where no automation is needed, a pre-programmed computing integrator may be sufficient. For higher control levels, a more intelligent device is necessary, such as a data station or minicomputer.
Methodology of Ion Exchange Chromatography:
The methodology of IEC involves the following steps:
- Selection of the Resin: Based on the target molecule’s charge and the pH of the working conditions, the appropriate resin (cation or anion exchanger) is selected.
- Column Preparation: The column is packed with the chosen resin and equilibrated with the buffer at the desired pH and ionic strength.
- Sample Loading: The sample is loaded onto the column, allowing ionic interactions between the target molecules and the resin.
- Washing: The column is washed with a buffer to remove unbound or loosely bound impurities.
- Elution: Elution is carried out by changing the buffer’s pH, ionic strength, or by introducing a competing ion, which helps to separate the bound analytes. Gradient elution is often employed for better separation.
- Detection: The separated ions are detected using various methods such as UV spectroscopy or conductivity.
- Regeneration: After use, the resin can be regenerated by washing with a strong acid or base to remove any remaining bound ions and then re-equilibrated with the buffer.
Applications of Ion Exchange Chromatography
- Protein Purification: Used in the purification of proteins, antibodies, and enzymes based on their charge properties.
- Water Treatment: Removal of undesirable ions (e.g., heavy metals) from water.
- Pharmaceuticals: Separation of charged drugs and their impurities.
- Amino Acid Analysis: Identification and quantification of amino acids in biological samples.
- Environmental Analysis: Used to monitor pollutants and toxic ions in the environment.
- Clinical Applications: Quantification of charged biomolecules such as vitamins, nucleotides, and DNA.

