Matter refers to anything that occupies space and has mass. It is the substance of the physical world, comprising particles at the atomic and molecular levels. Matter exists in various states—solid, liquid, gas, and plasma—each defined by the arrangement and behavior of its constituent particles. These particles include atoms, the fundamental building blocks of matter, and molecules, which consist of atoms chemically bonded together.
States of Matter
States of matter refer to the distinct forms that different phases of substances can take based on the arrangement and motion of their constituent particles. The primary states of matter are solid, liquid, gas, and plasma. Specific properties and behaviors characterize each state, and substances can transition between these states under varying conditions.
1. Solid
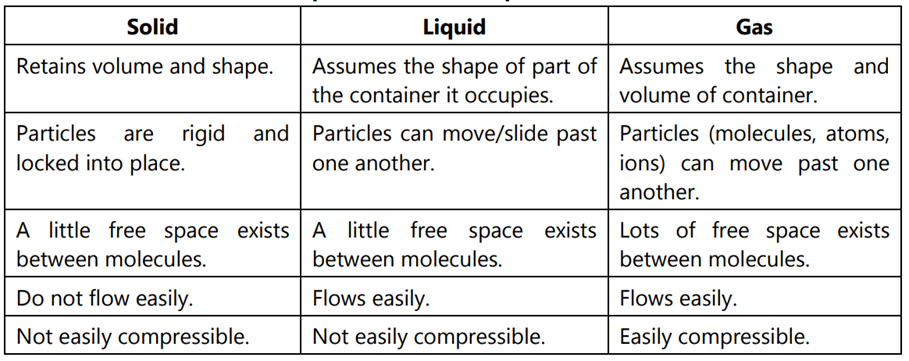
A solid is one of the fundamental states of matter characterized by its distinctive physical properties. Solids possess a fixed and definite shape, maintaining a specific geometric structure due to the closely packed and organized arrangement of particles. This organized structure contributes to the stability of solids. Also, like liquids, solids exhibit a definite volume-occupying a specific space. While the particles in a solid have fixed positions, they also display vibrational motion around their equilibrium points due to thermal energy. Solids include ice (solid water), wood, metal, and minerals. The properties of solids distinguish them from liquids and gases, playing a crucial role in numerous scientific, industrial, and everyday applications.
Characteristics
Definite shape and volume.
Particles are closely packed in a regular, fixed arrangement.
Vibrational motion within fixed positions.
Examples: Ice, wood, metal.
2. Liquid
A liquid, one of the fundamental states of matter, lacks a definite shape, taking on the form of its container. However, it maintains a definite volume, and its particles, though close together, exhibit a fluid and mobile structure, allowing liquids to flow. Examples like water and oil play crucial roles in diverse applications such as industrial processes and transportation, showcasing their adaptability. The incompressibility of liquids, ensuring a relatively constant volume under pressure, further contributes to their utility in various contexts.
Characteristics
Definite volume but no definite shape.
Particles are close together but not in a fixed arrangement.
Particles can move past each other, allowing the substance to flow.
Examples: Water, oil, mercury.
3. Gas
A gas, a fundamental state of matter, lacks a definite shape or volume, filling its container and taking its shape. Gases are highly compressible, with particles widely spaced and moving freely, altering volume under pressure. They exhibit expansibility, readily filling containers, and high diffusibility, mixing uniformly with other gases. Examples like oxygen and nitrogen play vital roles in scientific, industrial, and everyday applications, contributing to processes from combustion support to manufacturing.
Characteristics
No definite shape or volume.
Particles are far apart and move freely.
Rapid and random motion.
Examples: Oxygen, nitrogen, steam.
4. Plasma
Plasma is the fourth fundamental state of matter, distinct from solids, liquids, and gases. Unlike these traditional states, plasma does not have a definite shape or volume, filling the space it occupies. What sets plasma apart is the ionization of its particles—comprising positively charged ions and free electrons—which occurs at extremely high temperatures. This ionization imparts unique properties to plasma, including high electrical conductivity and a strong response to electromagnetic fields. Examples of plasma include the sun, stars, and various technological applications such as fluorescent lights and TVs. Understanding plasma is crucial in astrophysics, fusion research, and diverse technological advancements.
Characteristics
No definite shape or volume.
Consists of positively charged ions and free electrons.
Exists at extremely high temperatures.
Examples: Stars, lightning, and certain industrial processes.


1 thought on “Matter – States of Matter”