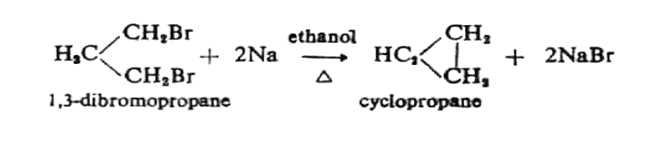
1. From Di-halogen Compounds: Suitable 1,3 or 1,4 di-halogen alkanes react with sodium or zinc to form cycloalkanes.
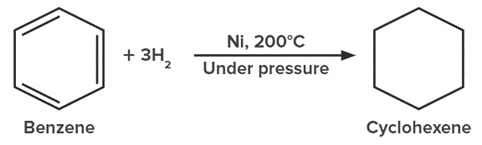
2. From Aromatic Compounds: Benzene can be converted into cyclohexane through catalytic hydrogenation at high temperature and pressure.
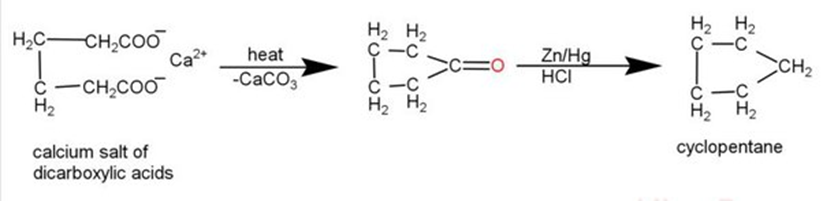
3. From Calcium or Barium salts of Dicarboxylic acids: Heating the calcium or barium salt of adipic, pimelic, or suberic acid produces a cyclic ketone. Clemmensen Reduction readily converts cyclic ketones into the corresponding cycloalkanes.
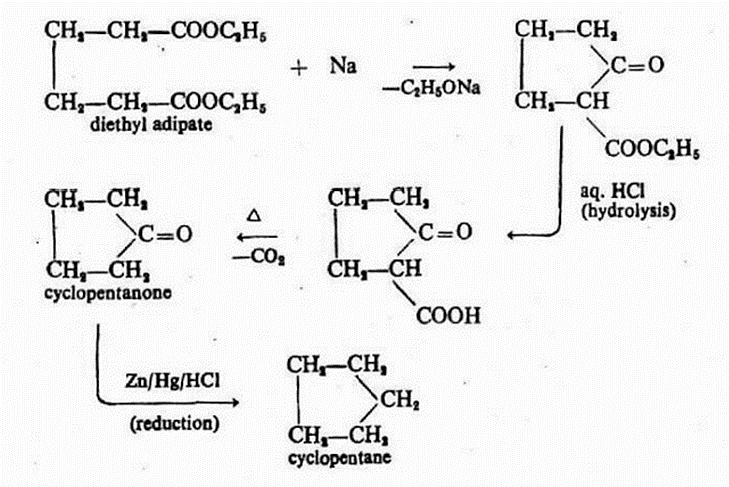
4. From Esters of Dicarboxylic acids (Dieckmann Reaction): When sodium is applied to the diester derived from adipic, pimelic, or suberic acid, an intramolecular acetoacetic ester condensation takes place, leading to the creation of a keto-ester. Upon hydrolysis of this keto-ester, cyclic ketones are generated. These cyclic ketones can undergo reduction to produce the respective cyclo-ketones.
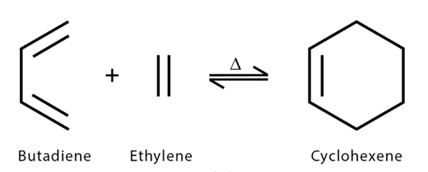
5. The Diels-Alder reaction: The Diels-Alder reaction is a chemical transformation resulting in the formation of a substituted cyclohexene derivative. It entails the interaction between a conjugated diene and a substituted alkene, referred to as the dienophile. This reaction leads to the synthesis of a six-membered ring by combining a diene with a pi bond.