The Naranjo Algorithm, developed in 1981 by Naranjo et al., is a structured, questionnaire-based tool used to assess the probability that a drug caused an adverse drug reaction (ADR). It is widely used in pharmacovigilance and clinical research to standardize causality assessment.
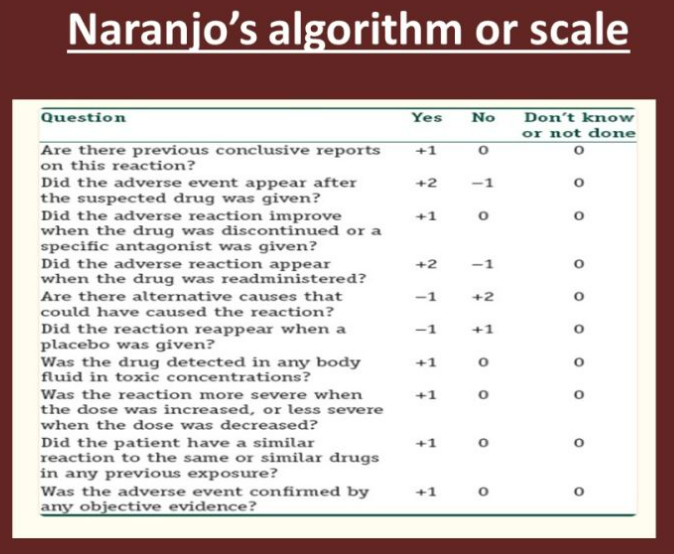
Structure of the Naranjo Algorithm
The Naranjo algorithm consists of 10 questions, each with a score of +1, 0, or -1 based on objective criteria. The total score determines the likelihood of causality.
| Question | Yes (+1) | No (0) | Don’t know (0) |
| 1. Are there previous conclusive reports on this reaction? | +1 | 0 | 0 |
| 2. Did the ADR appear after the suspected drug was administered? | +2 | -1 | 0 |
| 3. Did the ADR improve when the drug was discontinued (dechallenge)? | +1 | 0 | 0 |
| 4. Did the ADR reappear when the drug was readministered (rechallenge)? | +2 | -1 | 0 |
| 5. Are there alternative causes that could have caused the reaction? | -1 | +2 | 0 |
| 6. Did the ADR appear with a placebo? | -1 | 0 | 0 |
| 7. Was the drug detected in blood (therapeutic levels)? | +1 | 0 | 0 |
| 8. Was the ADR dose-dependent (i.e., higher dose = stronger reaction)? | +1 | 0 | 0 |
| 9. Did the patient have a similar reaction to this drug before? | +1 | 0 | 0 |
| 10. Was the ADR confirmed by objective evidence (e.g., biopsy, lab test)? | +1 | 0 | 0 |
Causality Categories and Scoring
The total Naranjo Score is used to classify the ADR into one of four categories:
| Total Score | Causality Classification | Interpretation |
| ≥ 9 | Definite | Strong evidence that the drug caused the ADR. |
| 5 – 8 | Probable | ADR is likely due to the drug, but other causes are possible. |
| 1 – 4 | Possible | The ADR may be due to the drug, but alternative explanations exist. |
| ≤ 0 | Doubtful | No strong evidence linking the ADR to the drug. |
Example Cases Using the Naranjo Algorithm
Example 1: Penicillin-Induced Anaphylaxis
Revised Example 1: Penicillin-Induced Anaphylaxis
A 30-year-old patient is given penicillin and develops severe anaphylaxis within minutes.
| Naranjo Question | Response | Score |
| 1. Are there previous conclusive reports on this reaction? | Yes | +1 |
| 2. Did the ADR appear after the suspected drug was administered? | Yes | +2 |
| 3. Did the ADR improve when the drug was discontinued (dechallenge)? | Yes | +1 |
| 4. Did the ADR reappear when the drug was readministered (rechallenge)? | Yes | +2 |
| 5. Are there alternative causes (other than the drug) that could have caused the reaction? | No | +2 |
| 6. Did the ADR appear with a placebo? | No | 0 |
| 7. Was the drug detected in blood (evidence of drug presence)? | Not tested | 0 |
| 8. Was the ADR dose-dependent (higher dose = stronger reaction)? | No | 0 |
| 9. Did the patient have a similar reaction to this drug before? | Yes | +1 |
| 10. Was the ADR confirmed by objective evidence? | Yes | +1 |


Example 2: Ibuprofen-Induced Gastric Ulcer
A 50-year-old patient develops gastric ulcers after 2 months on ibuprofen for arthritis.
| Naranjo Question | Response | Score |
| 1. Are there previous conclusive reports on this reaction? | Yes | +1 |
| 2. Did the ADR appear after the suspected drug was administered? | Yes | +2 |
| 3. Did the ADR improve when the drug was discontinued (dechallenge)? | Yes | +1 |
| 4. Did the ADR reappear when the drug was readministered (rechallenge)? | Not tested | 0 |
| 5. Are there alternative causes (e.g., alcohol, smoking) that could have caused the reaction? | Yes (partially) | -1 |
| 6. Did the ADR appear with a placebo? | No | 0 |
| 7. Was the drug detected in blood (evidence of drug presence)? | Not tested | 0 |
| 8. Was the ADR dose-dependent (higher dose = stronger reaction)? | Yes | +1 |
| 9. Did the patient have a similar reaction to this drug before? | Yes | +1 |
| 10. Was the ADR confirmed by objective evidence (e.g., endoscopy)? | Yes | +1 |


Example 3: Metformin-Induced Nausea
A 55-year-old patient starts metformin for type 2 diabetes and develops nausea within 2 days.
| Naranjo Question | Response | Score |
| 1. Are there previous conclusive reports on this reaction? | Yes | +1 |
| 2. Did the ADR appear after the suspected drug was administered? | Yes | +2 |
| 3. Did the ADR improve when the drug was discontinued (dechallenge)? | Not tested | 0 |
| 4. Did the ADR reappear when the drug was readministered (rechallenge)? | Not tested | 0 |
| 5. Are there alternative causes (e.g., diet, infection) that could have caused the reaction? | Yes (partially) | -1 |
| 6. Did the ADR appear with a placebo? | No | 0 |
| 7. Was the drug detected in blood (evidence of drug presence)? | Not tested | 0 |
| 8. Was the ADR dose-dependent (higher dose = stronger reaction)? | Not tested | 0 |
| 9. Did the patient have a similar reaction to this drug before? | No | 0 |
| 10. Was the ADR confirmed by objective evidence? | No | 0 |


Advantages of the Naranjo Algorithm
Standardized approach: Reduces subjectivity in ADR assessment.
Easy to use: Simple scoring system with objective questions.
Widely accepted: Used by regulatory authorities (FDA, WHO, EMA).
Limitations of the Naranjo Algorithm



Comparison: Naranjo Algorithm vs. WHO-UMC Causality System
| Feature | Naranjo Algorithm | WHO-UMC System |
| Approach | Questionnaire-based | Expert judgment-based |
| Scoring | Numeric (0–13) | Category-based (Certain, Probable, Possible, etc.) |
| Subjectivity | Less subjective | More subjective |
| Rechallenge Importance | Essential for high scores | Not always required |
| Use Case | Clinical trials, case reports | Regulatory pharmacovigilance |
Conclusion
The Naranjo Algorithm is a useful, structured method for determining whether a drug caused an adverse reaction. It is particularly effective for clinical case evaluations and research, whereas the WHO-UMC system is more suited for regulatory decision-making.
Strengths:
- Standardized scoring system makes it objective.
- Easy to apply, especially in clinical settings.
- Provides a probability score, aiding in decision-making.
Limitations:
- Not specific for all drug types and conditions.
- Lacks sensitivity in detecting complex interactions, such as those involving multiple drugs (polypharmacy).
- Relies on rechallenge and dechallenge, which may not be ethical or feasible in all situations.
Also visit to: Pharmacareerinsider.com

