The orbital picture of benzene provides a more accurate representation of its electronic structure than Kekulé’s model. In the orbital picture, resonance describes the distribution of pi electrons in the benzene ring. The key idea is that the pi electrons are delocalized and exist as clouds over all six carbon atoms, leading to a more stable and symmetric structure.
Orbital Picture of Benzene
1. Molecular Orbital Theory
The molecular orbital (MO) theory is used to describe the electronic structure of benzene. According to MO theory, a molecular orbital is formed by combining atomic orbitals on different atoms.
2. Formation of π Molecular Orbitals
In benzene, each carbon atom contributes one 2p orbital to forming six π molecular orbitals. Overlapping adjacent p orbitals form these orbitals and extend over the entire ring.
3. Delocalization of π Electrons
The six π electrons in benzene distribute over the six π molecular orbitals in the orbital picture. Unlike Kekulé’s model, which localizes electrons in specific single or double bonds, the orbital picture depicts electrons as delocalized, forming a cloud over the entire ring.
4. Resonance
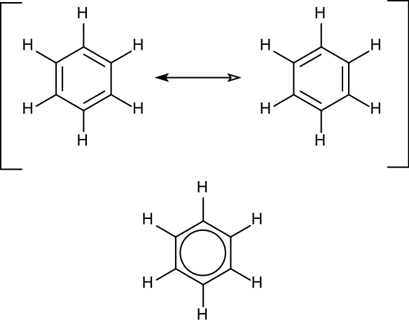
Resonance in benzene involves the idea that the electronic structure is a combination of a resonance hybrid of multiple contributing resonance structures. The location of the double bonds varies in each resonance structure, but the overall delocalization of pi electrons remains constant.
Resonance Structures:
5. Bond Lengths and Energies
All carbon-carbon bonds in benzene are identical in the orbital picture, with a bond order between a single and double bond. This leads to uniform bond lengths and intermediate bond energies, explaining benzene’s high stability.
6. Aromatic Stability
The delocalization of pi electrons in benzene results in aromatic stability, which is significantly greater than expected based on a localized double bond structure. The resonance energy associated with this delocalization contributes to the overall stability of benzene.
7. Aromaticity
Benzene is classified as an aromatic compound based on the presence of a planar, cyclic, and fully conjugated system of pi electrons. Aromatic compounds, according to the rules of aromaticity, exhibit enhanced stability.
The orbital picture provides a more accurate representation of benzene’s electronic structure, addressing the limitations of Kekulé’s model. The concept of resonance and the delocalization of pi electrons explain benzene’s stability, uniform bond lengths, and the absence of typical reactions observed in alkenes. This orbital perspective is essential in understanding the behavior of benzene and other aromatic compounds in organic chemistry.