Quantitative microscopy of crude drugs, particularly using the lycopodium spore method, is a valuable technique employed in pharmaceutical analysis to determine particle size distribution in powdered botanical materials. This method allows for the quantitative assessment of the size and size distribution of particles within a sample, which is crucial for evaluating the quality, authenticity, and consistency of herbal medicines and natural products. Let’s delve into a detailed note on the lycopodium spore method in quantitative microscopy of crude drugs:
Lycopodium Spore Method
Definition: The lycopodium spore method is a quantitative microscopy technique used to determine the particle size distribution in powdered drugs. It involves the addition of a known quantity of lycopodium spores, which have a uniform particle size, to the powdered drug sample. By comparing the number of spores to the number of drug particles observed under the microscope, the particle size distribution of the drug can be estimated.
Procedure
1. Preparation of Sample: A representative sample of the powdered drug is weighed accurately.
2. Preparation of Lycopodium Spores: A known quantity of lycopodium spores is weighed separately. Lycopodium spores are obtained from the spore mass of the club moss, Lycopodium clavatum.
3. Mixing of Samples: The weighed quantity of lycopodium spores is added to the powdered drug sample, and the mixture is thoroughly mixed to ensure homogeneity.
4. Microscopic Examination: A small quantity of the mixed sample is placed on a glass slide and covered with a cover slip. The slide is then examined under a microscope.
5. Counting and Analysis: Using a calibrated eyepiece reticle or stage micrometer, the number of lycopodium spores and drug particles within a defined area of the slide is counted. By comparing the counts, the particle size distribution of the drug sample can be calculated.
Calculation of Particle Size Distribution:
The particle size distribution of the drug sample can be calculated using the following formula:
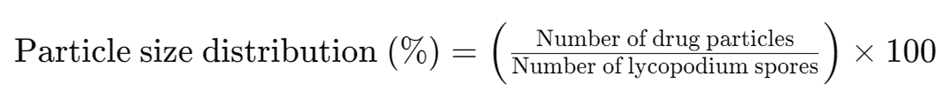
Importance
1. Quality Assessment: The lycopodium spore method provides valuable information about the particle size distribution of powdered drugs, which is essential for assessing their quality, uniformity, and consistency.
2. Dosage Formulation: Knowledge of particle size distribution is crucial for formulating dosage forms such as tablets, capsules, and powders, as it can influence drug dissolution, bioavailability, and stability.
3. Standardization: This method facilitates the standardization of herbal medicines by providing quantitative data on particle size distribution, ensuring consistency in composition and potency from batch to batch.
4. Regulatory Compliance: Many pharmacopoeias and regulatory authorities recommend the lycopodium spore method for the quantitative analysis of powdered drugs, making it an essential tool for quality control and compliance with pharmaceutical standards.
Limitations
1. Sample Preparation: Proper sample preparation is crucial to ensure homogeneity and accurate results. Inadequate mixing of lycopodium spores with the drug sample can lead to inaccuracies.
2. Subjectivity: The accuracy of particle counting and analysis may vary depending on the skill and experience of the analyst. Standardized procedures and training are essential to minimize subjectivity.
3. Interference: Certain factors such as agglomeration of particles, presence of impurities, and variations in particle shape may affect the accuracy of results and interpretation.
Conclusion
The lycopodium spore method is a valuable technique in quantitative microscopy of crude drugs, providing important information about particle size distribution for quality assessment, standardization, and regulatory compliance. Despite its limitations, when performed accurately and under standardized conditions, this method offers reliable results essential for ensuring the quality and efficacy of powdered herbal medicines and natural products.

