Raoult’s Law is a fundamental principle in the study of ideal solutions, providing insights into the behavior of mixtures, particularly liquids. Named after French chemist François-Marie Raoult, this law describes the relationship between the vapor pressures of the components in an ideal solution. Ideal solutions are theoretical models where the interactions between different components mirror those within each pure component.
Key Principles
1. Statement of Raoult’s Law:
Raoult’s Law states that the vapor pressure of a component in an ideal solution is directly proportional to its mole fraction in the mixture.
For a binary solution with components A and B:
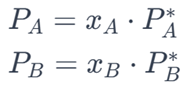
PA and PB are the partial vapor pressures of A and B, respectively. xA and xB are their mole fractions, and PA and PB are the vapor pressures of pure A and B.
2. Vapor Pressure and Mole Fraction:
The law implies that the total vapor pressure of the solution (Ptotal) is the sum of the partial vapor pressures of its components:
Ptotal = PA + PB
3. Conditions for Ideal Solutions:
Ideal solutions are characterized by the absence of significant intermolecular forces between different components.
Deviation from ideal behavior is more likely when the molecular interactions differ from those within each pure component.
4. Zero Deviation from Ideality:
In ideal solutions, there is zero deviation from Raoult’s law. This means that experimental vapor pressure values match those predicted by Raoult’s law.
Applications and Importance
1. Prediction of Vapor Pressure:
Raoult’s Law is instrumental in predicting the vapor pressures of individual components in mixtures, aiding in various industrial and scientific applications.
2. Distillation and Separation:
Understanding Raoult’s Law is crucial in distillation processes where vapor-liquid equilibrium is a key factor. It helps in separating components based on their differing vapor pressures.
3. Drug Formulation:
In pharmaceuticals, Raoult’s Law is considered in drug formulation, especially when dealing with solutions involving multiple components.
4. Chemical Engineering:
Chemical engineers use Raoult’s Law in designing and optimizing processes involving liquid mixtures, contributing to the efficiency of separation techniques.
Limitations
1. Deviation in Real Solutions:
Many real solutions deviate from Raoult’s Law due to non-ideal interactions, leading to the formation of non-ideal or real solutions.
2. Temperature and Pressure Dependence:
Raoult’s Law assumes constant temperature and pressure, and deviations become more pronounced under varying conditions.
Raoult’s Law serves as a valuable tool in understanding the behavior of ideal solutions and is foundational in the study of mixtures in various scientific and industrial fields. While deviations from ideality are common in real solutions, Raoult’s Law provides a useful framework for predicting and analyzing the behavior of components in idealized scenarios.

