Solubility, originating from the Latin word ‘solvere,’ meaning to dissolve, refers to the capability of a substance to dissolve in another under specific conditions completely. Typically expressed as the mass of solute per unit volume of solvent, the concentration of a solution is commonly measured in grams per liter. Solubility indicates the grams of solute required to saturate 100 grams or mL of the solvent at a specific temperature. In most instances, solutions involve solids dissolving in liquids, where the solid is the solute and the liquid is the solvent. The solute is the less abundant agent, while the solvent is the more abundant part of the solution. Solubility categorizes solids as soluble or insoluble in a liquid. As more solids are added to a liquid, the solution becomes more concentrated.
Solubility determines achievable concentration, with intrinsic solubility being a fixed value independent of environmental factors, while apparent solubility varies with pH and ionic strength. Factors like solvent type, particle size, stirring speed, and temperature affect solubility rates. In pharmaceuticals, solubility is crucial for dosage form preparation, as a drug needs to be in solution for absorption and biological activity. Control of environmental conditions is vital for influencing various solutions.
Solubility expressions
Solubility expressions are mathematical representations that quantify the degree to which a solute can dissolve in a given solvent under specific conditions. These expressions help chemists and researchers accurately describe and measure the solubility of substances. Here are some common solubility expressions and their explanations:
1. Percent by Mass (% m/m)
Percent by mass (% m/m) is a solubility expression that quantifies the mass of a solute in a solution relative to the total mass of the solution. It is calculated using the following formula:
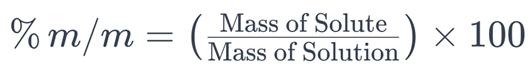
In this formula:
– (Mass of Solute) is the mass of the dissolved substance in the solution.
– (Mass of Solution) is the total mass of the solution, including both the solvent and the solute.
The result is multiplied by 100 to express the value as a percentage.
For example, if you have 5 grams of salt dissolved in a solution with a total mass of 100 grams, the percent by mass of the salt would be:
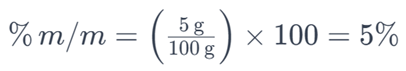
This means that the solution contains 5% of salt by mass. Percent by mass is a common way to express the concentration of solutes in various solutions.
2. Percent by Volume (% v/v)
The solubility expression used to quantify the volume of a solute in a solution relative to the total volume of the solution is percent by volume (% v/v). It is calculated using the following formula:
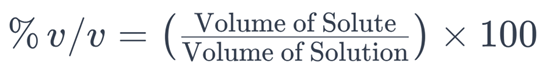
In this formula:
– (Volume of Solute) is the volume of the dissolved substance in the solution.
– (Volume of Solution) is the total volume of the solution, including both the solvent and the solute.
The result is multiplied by 100 to express the value as a percentage.
For example, if you have 30 milliliters of ethanol dissolved in a solution with a total volume of 150 milliliters, the percent by volume of ethanol would be:
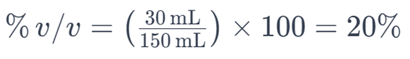
This means that the solution contains 20% ethanol by volume. Percent by volume is commonly used to express the concentration of solutes, especially in liquid-liquid solutions.
3. Molality (m)
Molality (m) quantifies the concentration of a solute in a solution relative to the mass of the solvent. It is defined as the number of moles of solute per kilogram of solvent, and the formula for molality is:

In this formula:
– (Moles of Solute) is the amount of solute in moles.
– (Mass of Solvent) is the mass of the solvent in kilograms.
Active voice: Molality provides a temperature-dependent measure of concentration and proves particularly useful when dealing with solutions undergoing temperature changes. It remains unaffected by temperature-dependent alterations in the volume of the solvent. Unlike other concentration measures, such as molarity, which depend on the total volume of the solution, molality remains constant regardless of temperature variations.
For example, if you have 0.5 moles of sodium chloride (NaCl) dissolved in 0.1 kilograms of water, the molality of the solution would be:
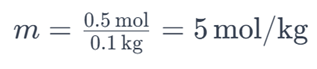
This indicates that the solution has a molality of 5 mol/kg.
4. Molarity (M)
Active voice: The solubility expression used to quantify the concentration of a solute in a solution in relation to the volume of the solution is Molarity ((M)). It is defined as the number of moles of solute per liter of solution, and the formula for molarity is:

In this formula:
– (Moles of Solute) is the amount of solute in moles.
– (Volume of Solution) is the total volume of the solution in liters.
Molarity is a common and widely used measure of concentration in chemistry. Unlike molality, which is temperature-dependent and based on the mass of the solvent, molarity depends on the total volume of the solution. It is important to note that temperature changes can affect the solution’s volume, leading to molarity variations.
For example, if you have 0.5 moles of sodium chloride (NaCl) dissolved in 0.5 liters of water, the molarity of the solution would be:
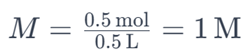
This indicates that the solution has a molarity of 1 M.
5. Mole Fraction (x)
Mole fraction (x) is a solubility expression representing the ratio of the moles of a specific component (solute or solvent) to the total number of moles in a solution. It is dimensionless and measures the relative abundance of a component within the solution. The formula for mole fraction is given by:

In this formula:
– (Moles of Component) is the number of moles of the specific component (solute or solvent).
– (Total Moles in Solution) is the sum of moles of all components present in the solution.
The mole fraction can range from 0 to 1, with 0 indicating the absence of the component and 1 indicating that the component is the only species present.
For example, in a solution containing 2 moles of glucose (C6H12O6) and 3 moles of water (H2O), the mole fraction of glucose would be:
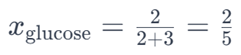
Similarly, the mole fraction of water would be:
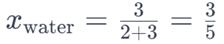
These mole fractions indicate the relative proportions of glucose and water in the solution.
6. Parts per Million (ppm)
Parts per million (ppm) is a solubility expression that quantifies the concentration of a particular component (usually a solute) in a solution. It is defined as the number of parts by weight of the solute per million parts by weight of the solution. The formula for calculating parts per million is as follows:

In this formula:
– (Mass of Solute) is the mass of the solute in grams.
– (Total Mass of Solution) is the total mass of the solution in grams.
The result is multiplied by (106) to express the concentration in parts per million.
For example, if a solution contains 2 grams of salt (NaCl) dissolved in 1,000 grams (1 kilogram) of water, the parts per million of salt in the solution would be:
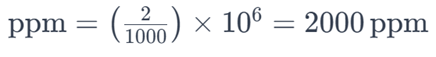
This means there are 2,000 parts of salt for every one million parts of the solution. Parts per million is convenient for expressing very small concentrations, especially in environmental and analytical chemistry contexts.
These solubility expressions provide versatile ways to characterize and communicate the concentration of solutes in solutions. The choice of expression depends on the specific requirements of the analysis or experimental conditions.
| Term | Required to dissolve one mass part of solute |
| Very soluble | <1 |
| Freely soluble | 1 to 10 |
| Soluble | 10 to 30 |
| Sparingly soluble | 30 to 100 |
| Slightly soluble | 100 to 1000 |
| Very slightly soluble | 1000 to 10,000 |
| Practically insoluble | ≥ 10,000 |

