The process of solvation, also known as dissolution, is a kinetic phenomenon that involves the attraction and association of molecules of a solvent with molecules or ions of a solute. This process is quantified by its rate, representing the speed at which it occurs. When a specific solvent dissolves a solute, solvent molecules surround and disperse the molecules or ions of the solute.
Solvation Complex
When a molecule or ion of solute interacts with a solvent, it forms a solvation complex, leading to a rearrangement of solvent and solute molecules. This complex is crucial for evenly distributing solute molecules within the solvent.
Forces in Solvation
Solvation is influenced by hydrogen bonding and van der Waals forces, including dipole-dipole, dipole-induced dipole, and induced dipole-induced dipole interactions. The specific forces at play depend on the molecular structure and properties of the solvent and solute.
Insoluble Solute Molecules
In cases where solute molecules are insoluble, they interact with each other instead of breaking apart and becoming solvated by the solvent. An example is the solvation of functional groups on the surface of an ion-exchange resin.
Solvation and Stabilization
Solvation is an interaction between a solute and the solvent, stabilizing the solute species in the solution. When water is the solvent, the term “hydration” is used.
Distinction from Solubility
Solvation is conceptually distinct from solubility. Solubility defines the dynamic equilibrium state when the dissolution rate matches the precipitation rate. The units used for these processes clarify the distinction, with the dissolution rate typically measured in mol/sec and solubility expressed as concentration (mass per volume or molarity).
Similarity between Solvent and Solute
The success of solvation depends on the similarity between the solvent and solute. “Like dissolves like,” meaning polar solutes dissolve in polar solvents, and non-polar solutes dissolve in non-polar solvents.
Solvation is a dynamic process driven by molecular interactions, leading to the formation of solvation complexes and the even solute distribution in the solvent. The forces involved, the nature of the solvent and solute, and the distinction from solubility contribute to the comprehensive understanding of this essential chemical phenomenon.
Association
Association or ion association is a chemical process in which ions with opposite electrical charges combine in a solution to create a unique chemical entity. Ion associates are categorized based on the number of ions that form associations, such as ion pairs or ion triplets. Ion pairs can further be classified by their interaction, distinguishing between contact, solvent-shared, or solvent-separated types. The dielectric constant of the solvent is a crucial factor influencing the degree of ion association. Vibrational spectroscopy is commonly used to characterize ion associates in these chemical reactions.
Ion pairs are formed when a cation and anion come together:
An+ + Bm− ⇌ AB(n−m)+
There are three distinct types of ion pairs depending on the extent of solvation of the two ions:
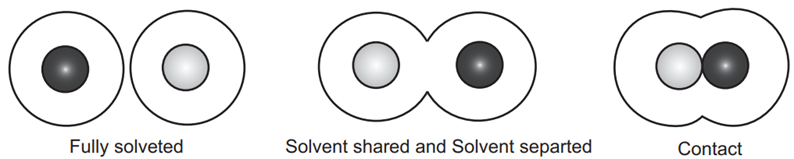
Ion Interaction in Solution: Understanding the Dynamics
Schematic Representation:
- The depiction showcases ions in a solution, utilizing circles to symbolize spheres of varying sizes.
- Cations are visually distinguished with a dark color, while anions are represented in grey.
Solvent Shell and Ion Pair Formation:
- The space around ions serves as a visual representation of solvent molecules residing within the primary solvation shell, with secondary solvation intentionally excluded.
- A fully solvated ion pair is characterized by the presence of complete primary solvation spheres for both ions.
- Contrastingly, a solvent-shared ion pair arises when approximately one solvent molecule resides between the cation and anion.
- In the case of a contact ion pair, ions are in direct contact, and the specifics of their solvation shell are generally unknown.
Aqueous Solutions:
- In aqueous solutions, metal cations exhibit a tendency to be surrounded by 4 to 9 solvent molecules within the primary solvation shell.
- The nature of solvation for anions in such solutions remains largely unidentified.
Complex Nomenclature:
- A solvent-shared ion pair is alternatively known as an outer-sphere complex. This term finds common usage in coordination chemistry, describing a complex formed between a solvated metal cation and an anion.
- Similarly, a contact ion pair may be referred to as an inner-sphere complex. The primary distinguishing factor lies in the proximity of ions, with the order being fully solvated > solvent-shared > contact.
Interaction Dynamics:
- Interactions in fully solvated and solvent-shared ion pairs predominantly exhibit electrostatic characteristics.
- In contrast, a contact ion pair demonstrates some covalent character in the bond between the cation and anion.
Ion Aggregates and Ternary Associates:
- Ion triplets can form from one cation and two anions or vice versa.
- Higher aggregates, such as a tetramer (AB)4, are within the realm of possibility.
- Ternary ion associates involve the association of three species, contributing to the complexity of ion interactions.
Intrusion Ion Pair:
- Another intriguing type, known as an intrusion ion pair, has been characterized in the study of ion interactions, adding further depth to our understanding.




