The absorption spectrum of a molecule represents how it absorbs light across various wavelengths. The environment of a molecule, particularly the solvent it is dissolved in, plays a crucial role in influencing its electronic transitions and, consequently, its absorption spectrum. The solvent can shift the absorption maxima, alter the intensity of absorption bands, and even affect the shape of the spectrum. This phenomenon arises due to various solvent-molecule interactions.
Basics of Absorption Spectra
Electronic Transitions: Absorption of light excites electrons from the ground state (usually the HOMO, highest occupied molecular orbital) to an excited state (typically the LUMO, lowest unoccupied molecular orbital).
Absorption Wavelength: The energy difference (ΔE) between these states determines the wavelength (λ) of absorption (λ = hc/ΔE).
Impact of Solvent: The solvent can modulate (ΔE) through solute-solvent interactions, resulting in shifts in the spectrum.
Types of Solvent Effects
(A) Bathochromic (Red) Shift: A shift of the absorption maximum (λmax) to a longer wavelength (lower energy).
Cause:
- Stabilization of the excited state more than the ground state.
- Polar solvents interacting with polar or charge-separated excited states.
Example: Transition from ( π → π∗) in aromatic compounds is bathochromically shifted in polar solvents.
(B) Hypsochromic (Blue) Shift: A shift of λmax to a shorter wavelength (higher energy).
Cause:
- Greater stabilization of the ground state relative to the excited state.
- Occurs when hydrogen-bonding solvents interact predominantly with the ground state.
Example: N → π∗ transitions in carbonyl compounds often exhibit a blue shift in protic solvents.
(C) Hyperchromic Effect: An increase in the intensity (absorbance) of a spectral band.
Cause: Enhanced transition probability due to solvent-induced changes in electronic distribution.
(D) Hypochromic Effect: A decrease in the intensity of a spectral band.
Cause: Reduced transition probability or aggregation effects in the solvent.
Factors Influencing Solvent Effects
(A) Polarity of the Solvent
Dipole Moment: Polar solvents stabilize polar excited states.
Dielectric Constant(ϵ): High- ϵ solvents reduce Coulombic interactions, affecting \(\Delta E\).
(B) Hydrogen Bonding
Protic Solvents: Form hydrogen bonds with solute molecules, affecting electronic states.
Affects n → π∗ Transitions: These transitions are more susceptible to hydrogen bonding.
(C) Polarizability of the Solvent
Induced Dipole Interactions: Non-polar but polarizable solvents can stabilize electronic states through dispersion forces.
(D) Specific Solvent-Solute Interactions
Charge Transfer: Solvents influence donor-acceptor interactions, altering charge transfer bands.
Chemical Reactions: Solvent-induced tautomerism or complex formation can modify spectra.
Quantitative Description of Solvent Effects
(A) Linear Solvation Energy Relationships (LSER)
Equation: νmax = ν0 + aα + bβ + cπ∗
νmax : Wavenumber of absorption maximum.
α: Solvent hydrogen bond donor (HBD) ability.
β: Solvent hydrogen bond acceptor (HBA) ability.
π: Solvent polarity/polarizability.
Purpose: Quantifies the contributions of various solvent properties to spectral shifts.
(B) Lippert-Mataga Equation
Application: Describes solvent-induced shifts based on polarity.
Equation:
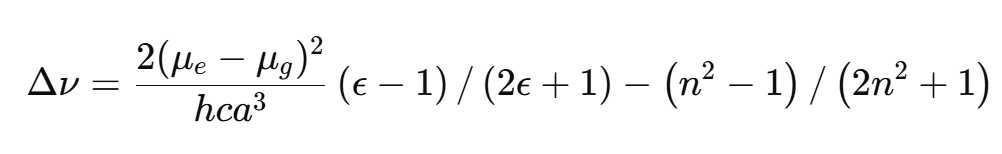
Δν: Spectral shift.
μe,μg: Dipole moments of excited and ground states.
ϵ,n: Dielectric constant and refractive index of the solvent.
a: Radius of the solute molecule.
Practical Applications
(A) Tuning Optical Properties: Solvent effects are used to optimize the light absorption/emission of dyes and pigments for lasers, LEDs, and sensors.
(B) Determination of Molecular Polarity: Solvent shifts help estimate dipole moments of ground and excited states.
(C) Pharmaceuticals and Biochemistry: Understanding solvent effects is crucial for studying drug solubility, protein-ligand interactions, and enzyme mechanisms.
Experimental Considerations
Choice of Solvent: Use solvents that do not chemically react with the solute.
Concentration: High concentrations may lead to aggregation, affecting spectral shape.
Temperature: Solvent polarity and viscosity can vary with temperature, influencing spectra.
Conclusion
The solvent effect on absorption spectra is a complex interplay of physical and chemical interactions between the solute and solvent. By understanding these effects, researchers can tailor experimental conditions, interpret spectral data, and design molecules with desired optical properties.

