Microbial contaminants can originate from various sources, impacting the safety and quality of pharmaceuticals, food products, and other environments. The primary sources of microbial contamination include the environment, raw materials, equipment, personnel, and packaging materials.
1. Environmental Sources: Air, water, and soil serve as major environmental reservoirs for microorganisms. Airborne microbes, such as fungi and bacteria, are carried by dust particles or aerosols, leading to contamination during manufacturing and storage. Water, if not properly treated, may harbor bacteria like Pseudomonas and E. coli, which can cause contamination in water-based products.
2. Raw Materials: Raw materials used in the production of pharmaceuticals or food products are often exposed to microbes during harvesting, transportation, or storage. Plant-based raw materials can be contaminated with fungi, whereas animal-derived products are prone to bacterial contamination, including Salmonella and Listeria.
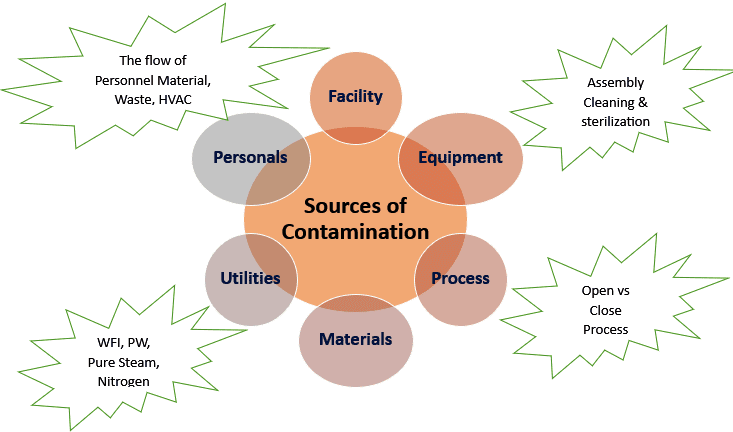
3. Equipment and Manufacturing Tools: Inadequate cleaning of equipment can lead to biofilm formation, which harbors a variety of microorganisms, including Staphylococcus aureus, Pseudomonas aeruginosa, and other resistant strains. Poor maintenance of equipment or improper sterilization techniques are common sources of contamination.
4. Personnel: Human operators are significant vectors for microbial contamination. Skin, hair, and respiratory secretions can introduce bacteria like Staphylococcus, Corynebacterium, or fungi such as Candida into sterile environments. Inadequate handwashing or improper use of protective equipment can exacerbate this risk.
5. Packaging Materials: Contaminated packaging materials can introduce bacteria and fungi into products, especially when materials are exposed to humid conditions during storage. Packaging surfaces can also carry dust and airborne spores, leading to contamination.
Types of Microbial Contaminants
Microbial contaminants can be broadly classified into the following types:
1. Bacteria: Bacteria are the most common contaminants. Gram-positive bacteria such as Staphylococcus aureus and Gram-negative bacteria like Pseudomonas aeruginosa and Escherichia coli are frequently found in contaminated products. These microbes can lead to spoilage, degradation of products, and pose health risks if ingested or applied.
2. Fungi: Molds and yeasts are major fungal contaminants. Common molds include Aspergillus, Penicillium, and Rhizopus, while yeasts such as Candida and Saccharomyces are also found. Fungal contamination is often associated with spoilage, especially in products with higher water activity, and can result in the production of mycotoxins.
3. Viruses: Although less common, viral contamination can occur, especially in biological products like vaccines or tissue-derived pharmaceuticals. Human viruses, such as hepatitis or influenza viruses, may be introduced through contaminated human material or improper handling.
4. Spores: Bacterial and fungal spores are particularly resilient and can withstand adverse conditions. Spores from bacteria such as Bacillus or Clostridium can survive sterilization processes and later germinate under favorable conditions, leading to contamination and spoilage.
Assessment of Microbial Contamination
Assessing microbial contamination involves identifying and quantifying the types and levels of microorganisms present in a product or environment. This can be achieved through several methodologies:
1. Bioburden Testing: This is a routine method used to assess the total microbial load in a product or material. Bioburden testing involves culturing microorganisms on a nutrient medium and quantifying the colony-forming units (CFUs) after incubation. It is commonly applied in pharmaceutical and medical device industries to ensure compliance with sterility standards.
2. Sterility Testing: Sterility testing is used specifically for products intended to be sterile, such as injectables, surgical instruments, or ophthalmic solutions. The product is incubated in growth media to determine the absence or presence of viable microorganisms.
3. Endotoxin Testing: Endotoxins, often produced by Gram-negative bacteria, can be detected using the Limulus Amebocyte Lysate (LAL) test. This test is critical for assessing pyrogenic substances in parenteral products to prevent adverse reactions.
4. Environmental Monitoring: Regular monitoring of manufacturing environments is crucial in identifying potential sources of contamination. Air sampling, surface swabs, and water testing are commonly used to detect microbial load in clean rooms and controlled environments.
5. Rapid Microbial Methods: Advances in technology have led to the development of rapid microbial detection methods such as PCR (Polymerase Chain Reaction) and flow cytometry. These techniques allow for faster detection and identification of microbial contaminants compared to traditional culturing methods.
Microbial Spoilage and Its Impacts
Microbial spoilage refers to the degradation of products due to microbial activity. It is a significant concern in industries like pharmaceuticals, food, and cosmetics, as spoilage can lead to product rejection, financial losses, and potential health hazards. The mechanisms of spoilage depend on the type of contaminant and the nature of the product:
1. Bacterial Spoilage: Bacteria can cause spoilage by producing enzymes that degrade proteins, fats, and carbohydrates. This results in off-odors, discoloration, and texture changes in products. For example, Pseudomonas species are known to produce lipases and proteases, causing rancidity in food products.
2. Fungal Spoilage: Fungi can grow on products with higher moisture content and produce visible mold, as well as secondary metabolites like mycotoxins, which can be harmful to humans. Molds such as Aspergillus and Penicillium are notorious for spoiling grains, fruits, and other perishable items.
3. Physical and Chemical Deterioration: Microbial contamination can lead to changes in pH, oxidation-reduction potential, and the release of gases, all of which contribute to physical and chemical spoilage. This is particularly detrimental in pharmaceutical products, where even slight changes in chemical composition can reduce efficacy or increase toxicity.
Conclusion
Microbial contamination is an omnipresent risk across industries that deal with consumable and sterile products. Understanding the sources and types of microbial contaminants, assessing contamination levels, and controlling spoilage are essential for maintaining product safety and quality. The implementation of proper hygiene practices, environmental monitoring, and effective sterilization techniques are crucial in mitigating contamination risks and ensuring compliance with regulatory standards.

