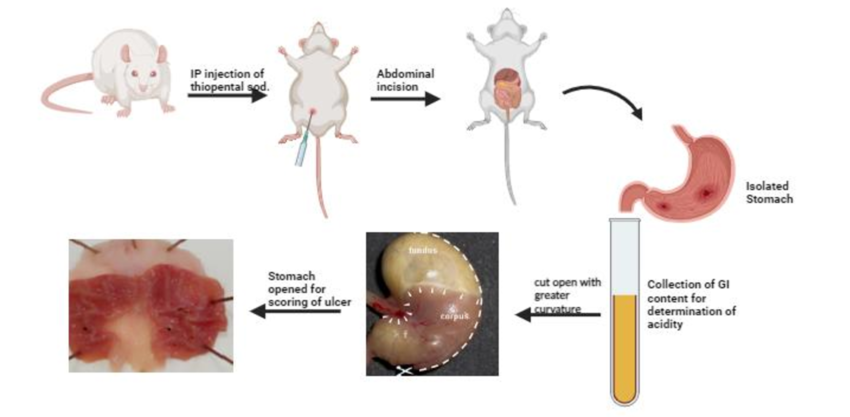
Aim: Study of anti-ulcer activity using pylorus ligation (shay) rat model and nsaid-induced ulcer model
References
- Shay, H., Komarov, S. A., Fels, S. S., Meranze, D., Gruenstein, M., & Siplet, H. (1945). A simple method for the uniform production of gastric ulceration in the rat. Gastroenterology, 5, 43-61.
- Parmar, N. S., & Desai, J. K. (1993). A review of the current methodology for the evaluation of gastric and duodenal anti-ulcer agents. Indian Journal of Pharmacology, 25, 120-135.
- Goel, R. K., & Bhattacharya, S. K. (1991). Gastroduodenal mucosal defense and mucosal protective agents. Indian Journal of Experimental Biology, 29, 701-714.
1. Objective
To evaluate the anti-ulcer activity of a test drug using the Pylorus Ligation (Shay) Rat Model and NSAID-Induced Ulcer Model by assessing ulcer formation, gastric secretions, and ulcer index.
2. Principle
Peptic ulcers result from an imbalance between gastric acid secretion and mucosal protection mechanisms. The Pylorus ligation model leads to ulcer formation due to accumulated gastric acid, while NSAID-induced ulcers arise from inhibition of prostaglandins, leading to reduced mucosal protection. Evaluating a drug in these models helps determine its gastroprotective effects.
3. Materials Required
- Animals: Healthy Wistar rats (150–200 g)
- Reagents and Chemicals:
- Test compound (plant extract, synthetic drug, or standard anti-ulcer drug like omeprazole)
- Carboxymethyl cellulose (CMC) for drug suspension
- Normal saline (0.9%)
- Diethyl ether for anesthesia
- Pyloric ligation sutures
- NSAID (Indomethacin 20 mg/kg)
- 0.5% Alcian blue solution (for mucus estimation)
- 0.1% Toluidine blue solution
- Equipment:
- Syringes and oral gavage
- Surgical instruments (forceps, scissors, sutures)
- Dissecting microscope
- pH meter
- Centrifuge
- Microplate reader for biochemical estimations
4. Experimental Procedure
4.1. Pylorus Ligation (Shay) Rat Model
- Animal Grouping:
Control group: Received normal saline (1 mL/kg, p.o.)
Negative control group: Pylorus ligation only
Test groups: Pre-treated with different doses of the test drug (e.g., 10, 50, 100 mg/kg)
Standard group: Pre-treated with omeprazole (20 mg/kg, p.o.)
- Procedure:
- Fast rats for 24 hours but allow free access to water.
- Anesthetize the rat using diethyl ether.
- Perform a midline abdominal incision and identify the pyloric region.
- Ligate the pylorus carefully without damaging blood supply.
- Close the incision using sutures and allow the rat to recover.
- After 4 hours, sacrifice the rat using cervical dislocation.
- Collect gastric juice and measure its volume, pH, total acidity, and pepsin activity.
- Stomach is excised, washed, and examined for ulcers under a dissecting microscope.
4.2. NSAID-Induced Ulcer Model
- Animal Grouping: Same as for pylorus ligation model.
- Procedure:
- Fast rats for 24 hours before the experiment.
- Administer NSAID (Indomethacin 20 mg/kg, p.o.) to induce ulcers.
- Administer the test drug or standard drug 30 minutes before NSAID.
- After 6 hours, sacrifice the rats and examine the stomach for ulcers.
- Measure ulcer index, gastric pH, and mucus content.
5. Observations & Data Analysis
5.1. Ulcer Index Calculation
Ulcers are scored as follows:
- 0 = No ulcers
- 1 = Superficial ulcers
- 2 = Deep ulcers
- 3 = Perforated ulcers
5.2. Gastric Secretion Analysis
- Volume of gastric juice (mL)
- pH measurement using a pH meter
- Total acidity (mEq/L) determined by titration with NaOH
- Pepsin activity measured by protein digestion assay
5.3. Sample Data Table
| Group | Ulcer Index | Gastric pH | Total Acidity (mEq/L) | Mucus Content (mg/g) |
| Control | 0.00 | 3.5 | 10.2 | 120 |
| Pylorus Ligation | 4.5 | 2.0 | 35.6 | 80 |
| NSAID + Vehicle | 5.2 | 1.8 | 40.2 | 75 |
| Test Drug (10 mg/kg) | 3.8 | 2.8 | 28.5 | 95 |
| Test Drug (50 mg/kg) | 2.4 | 3.2 | 22.8 | 110 |
| Test Drug (100 mg/kg) | 1.2 | 3.8 | 15.6 | 130 |
| Omeprazole | 0.8 | 4.0 | 12.4 | 140 |
6. Interpretation of Results
- A higher ulcer index indicates severe ulceration.
- Increased gastric pH and reduced total acidity suggest gastroprotection.
- Higher mucus content implies enhanced mucosal defense.
- A dose-dependent reduction in ulcer severity confirms anti-ulcer activity.
7. Precautions
- Maintain sterile conditions during surgery.
- Avoid excess tissue damage while ligating the pylorus.
- Handle NSAIDs carefully as they can cause severe gastric mucosal damage.
- Euthanize animals humanely following ethical guidelines.
8. Conclusion
This study evaluates the potential anti-ulcer effects of a test drug using two validated experimental models. A reduction in ulcer index, increased gastric pH, and enhanced mucus content suggest significant gastroprotective properties.
Visit to: Pharmacareerinsider.com
