Sucrose, a disaccharide and a type of carbohydrate, comprises one molecule of glucose and one molecule of fructose linked together by an α,β-1,2-glycosidic bond. Sucrose, widely known as table sugar, is the most extensively consumed sugar by humans and is prevalent in many plants.
In its crystalline form, sucrose is a white, odorless, and sweet-tasting powder or solid. It plays a crucial role as a major source of dietary energy and finds frequent use as a sweetener in various foods and beverages. In the digestive system, sucrase, the enzyme, facilitates the hydrolysis of sucrose, breaking it down into its constituent monosaccharides, glucose, and fructose. The body can then absorb and utilize these for energy.
Structure of Sucrose
Represent as follows the structural formula for sucrose:
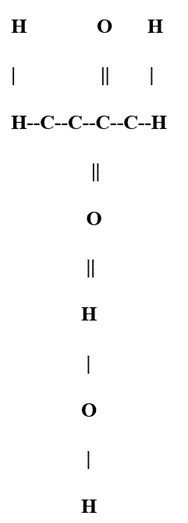
In this representation:
“C” represents a carbon atom.
“H” represents a hydrogen atom.
“O” represents an oxygen atom.
“||” represents the α,β-1,2-glycosidic bond.
Each vertical line between carbon atoms represents a single covalent bond.
This structure illustrates the linkage between the carbon atom at the first position of glucose and the carbon atom at the second position of fructose, forming the glycosidic bond. Sucrose, commonly known as table sugar, is present in various plants, particularly in sugar cane and sugar beets. During digestion, the enzyme sucrase breaks sucrose down into its constituent glucose and fructose molecules.

