Definition: A suspension is a biphasic liquid dosage form containing finely divided insoluble solid particles dispersed uniformly in a liquid medium. The solids are maintained in suspension by employing suitable suspending agents. Suspensions are used for oral, topical, ophthalmic, and parenteral delivery of drugs.
Characteristics of Suspensions
1. Heterogeneous System: Composed of two phases—dispersed phase (solid particles) and dispersion medium (liquid).
2. Particle Size: Typically ranges from 0.5 µm to 5 µm.
3. Settling: Particles tend to settle over time due to gravity; hence, uniformity before dosing is essential (shake well before use).
4. Stability: Requires stabilizing agents to prevent caking or flocculation.
Components of Suspensions
1. Active Ingredient (Dispersed Phase): The solid drug that is insoluble in the dispersion medium.
Example: Paracetamol, antacids.
2. Dispersion Medium: The liquid phase in which the solid particles are suspended.
Example: Water, oil.
3. Suspending Agents: Improve viscosity and maintain uniform dispersion of particles.
Example: Methylcellulose, xanthan gum, bentonite.
4. Wetting Agents: Reduce the surface tension between the solid particles and liquid, aiding dispersion.
Example: Polysorbates, glycerin.
5. Preservatives: Prevent microbial growth.
Example: Methylparaben, sodium benzoate.
6. Flavors and Sweeteners: Improve the palatability of oral suspensions.
Example: Sucrose, sorbitol.
7. Buffers and pH Adjusters: Maintain stability by optimizing pH.
Example: Citric acid, sodium citrate.
Advantages of Suspensions
1. Enhanced Bioavailability: Drugs in suspension may have better dissolution rates compared to tablets or capsules.
2. Flexibility of Dosage: Allows for dose adjustments by varying the volume administered.
3. Taste Masking: Insoluble drugs are easier to mask in suspension form.
4. Delivery of Insoluble Drugs: Enables administration of drugs that are poorly soluble in water.
Disadvantages of Suspensions
1. Physical Instability: Settling and caking of particles can occur over time.
2. Inconsistent Dosage: Non-uniform dispersion may lead to variable dosing.
3. Storage Issues: Sensitive to temperature changes and microbial contamination.
4. Palatability: May have a gritty texture.
Types of Suspensions
1. Oral Suspensions
Example: Antacids (magnesium hydroxide), antibiotics (amoxicillin).
2. Topical Suspensions
Example: Calamine lotion, sulfur suspensions for acne.
3. Ophthalmic Suspensions
Example: Tobramycin eye drops.
4. Parenteral Suspensions
Example: Steroid injections (methylprednisolone acetate).
5. Rectal Suspensions
Example: Barium sulfate for radiographic studies.
Formulation Considerations
1. Particle Size Control: Smaller particles provide a uniform suspension and improve stability.
2. Sedimentation Rate
Governed by Stokes’ Law:
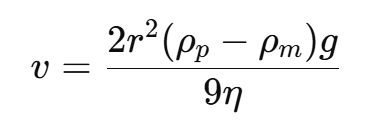
Where:
v: Sedimentation velocity.
r: Radius of the particle.
ρp,ρm : Densities of particle and medium.
g: Gravitational acceleration.
η: Viscosity of the medium.
3. Wettability: Ensured by wetting agents to avoid clumping.
4. Flocculation and Deflocculation
Flocculated suspensions prevent caking by forming loose aggregates that settle rapidly and can be redispersed easily.
Preparation Methods
1. Dispersion Method: Mixing the solid particles with a portion of the liquid to form a paste, followed by dilution to the required volume.
2. Precipitation Method: The drug is precipitated out of a solution to form fine particles.
3. Controlled Flocculation: Addition of electrolytes or polymers to control particle aggregation.
Evaluation of Suspensions
1. Sedimentation Volume (F)
Indicates the stability of the suspension.
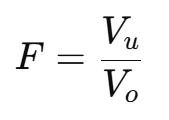
Where:
Vu: Volume of sediment.
Vo: Total volume of suspension.
2. Redispersibility: Measured by the ease of redistributing settled particles upon shaking.
3. Particle Size Distribution: Assessed using microscopy or particle size analyzers.
4. Rheological Properties: Viscosity measurement to ensure proper flow and stability.
5. pH Measurement: Ensures compatibility with the drug and prevents degradation.
Stability Issues
1. Sedimentation: Particles settle under gravity; can be reversed by shaking.
2. Caking: Formation of a hard, irreversible sediment.
3. Flocculation: Controlled flocculation can enhance redispersibility.
4. Aggregation: Particles stick together, leading to larger clusters.
5. Crystal Growth: Due to temperature fluctuations or Ostwald ripening.
Applications of Suspensions
1. Medicinal Use: Antacids (e.g., aluminum hydroxide), antibiotics (e.g., erythromycin).
2. Diagnostic Use: Barium sulfate suspensions for X-ray imaging.
3. Cosmetic Use: Suspensions in lotions or makeup products.
4. Veterinary Use: Oral or injectable suspensions for animals.
Examples of Suspensions
Oral: Amoxicillin suspension, ibuprofen suspension.
Topical: Calamine lotion.
Parenteral: Depo-Provera (contraceptive suspension).
Ophthalmic: Prednisolone acetate eye drops.
Suspensions are versatile dosage forms suitable for various routes of administration. Their formulation and stability depend on proper selection of components, particle size control, and stabilization methods. With advancements in technology, suspensions continue to play a vital role in drug delivery, particularly for insoluble drugs.

