Introduction
UV-Visible spectroscopy is an analytical technique that measures the absorption or reflectance of ultraviolet (UV) and visible light (200–800 nm) by a substance. It is widely used for quantitative and qualitative analysis of molecules in chemistry, biochemistry, and materials science.
Principle of UV-Visible Spectroscopy
The technique is based on the absorption of light energy, which promotes electrons in molecules from a ground state to an excited state. The specific wavelengths of light absorbed depend on the electronic structure of the molecule, typically involving π-π or n-π transitions.
Components of a UV Visible Spectrophotometer
1. Light Source: Produces UV (deuterium lamp) and visible light (tungsten-halogen lamp).
2. Monochromator: Separates light into its component wavelengths using a prism or diffraction grating.
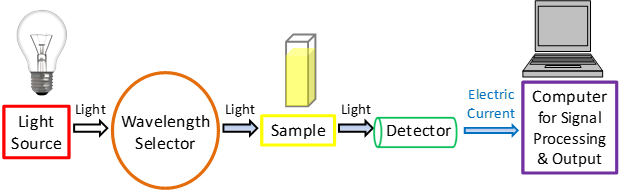
3. Sample Holder: A cuvette (usually quartz or glass) containing the sample solution.
4. Detector: Photodiodes or photomultiplier tubes that measure the intensity of transmitted light.
5. Data Processor: Converts the signal into an absorbance spectrum for analysis.
Working Mechanism
1. Light Emission: The spectrophotometer emits a beam of light covering UV and visible regions.
2. Wavelength Selection: The monochromator isolates the desired wavelength.
3. Sample Interaction: The light passes through the sample in the cuvette, and the absorbance is measured.
4. Intensity Comparison: The detector compares the transmitted light intensity (I) to the initial light intensity (I₀) to calculate absorbance using the formula:
A = −log (I/I0)
Or
A = εcl
where (ε) is the molar absorptivity, (c) is the concentration, and (l) is the path length.
Applications of UV-Visible Spectroscopy
1. Quantitative Analysis:
Determination of Concentration: Based on Beer-Lambert’s Law, UV-visible spectroscopy is used to determine the concentration of analytes in a solution.
Example: Analysis of drugs, dyes, and food additives.
2. Qualitative Analysis:
Identification of functional groups and structural information.
Example: Characterization of chromophores in organic compounds.
3. Kinetic Studies:
Monitoring reaction rates by observing changes in absorbance over time.
4. Pharmaceutical Applications:
Drug purity testing and dissolution studies.
5. Environmental Analysis:
Measurement of pollutants like nitrates and sulfates.
6. Biochemical Applications:
Protein quantification using aromatic amino acid absorption at 280 nm.
Nucleic acid quantification at 260 nm.
Advantages of UV-Visible Spectroscopy
Non-Destructive: The sample is preserved during analysis.
Rapid: Results are obtained quickly.
Versatile: Applicable to a wide range of analytes.
Limitations of UV-Visible Spectroscopy
Requires Transparent Samples: Turbid or highly colored solutions can interfere with accuracy.
Limited to Chromophores: Only molecules that absorb UV-visible light can be analyzed.
Environmental Sensitivity: Light source stability and solvent quality affect measurements.
Instrumentation Variants of UV-Visible Spectroscopy
1. Single-Beam Spectrophotometer: Measures one wavelength at a time.
2. Double-Beam Spectrophotometer: Splits light into two beams for sample and reference, ensuring more accurate measurements.
Data Interpretation
Absorption Spectrum: A plot of absorbance vs. wavelength. The λmax (wavelength at maximum absorbance) is characteristic of the molecule being analyzed.
Quantitative Calibration: A calibration curve is constructed using standards of known concentration, and unknown samples are analyzed against this curve.
Common Solvents of UV-Visible Spectroscopy
Water, ethanol, methanol: Used due to transparency in the UV-visible range.
Chloroform and hexane: Suitable for non-polar compounds.
UV-Visible spectroscopy is a cornerstone analytical method offering precision, sensitivity, and versatility. Its widespread application across scientific fields underscores its value in research, quality control, and innovation.

